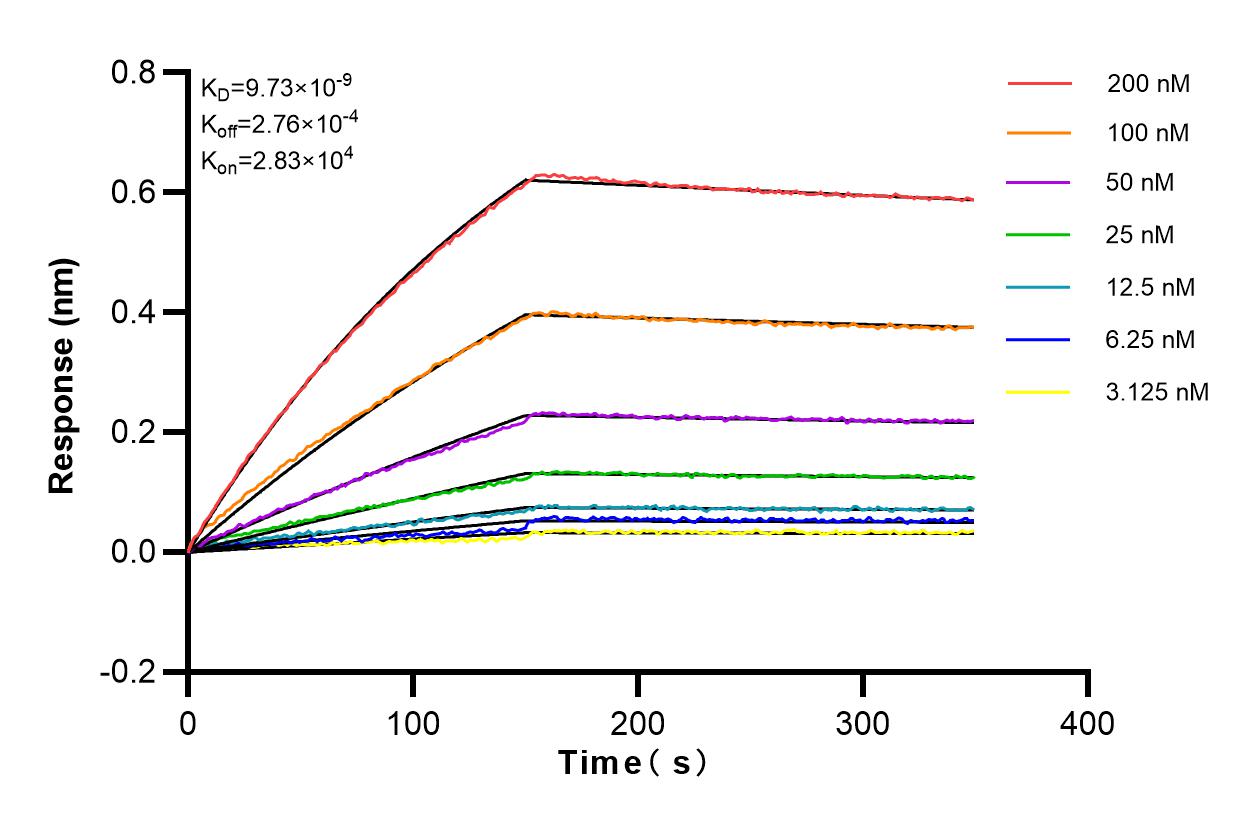
Validation Data Gallery
Tested Applications
Recommended dilution
| Application | Dilution |
|---|---|
| It is recommended that this reagent should be titrated in each testing system to obtain optimal results. | |
| Sample-dependent, Check data in validation data gallery. | |
Product Information
83077-4-RR targets STT3A in ELISA applications and shows reactivity with human samples.
| Tested Reactivity | human |
| Host / Isotype | Rabbit / IgG |
| Class | Recombinant |
| Type | Antibody |
| Immunogen |
CatNo: Ag31994 Product name: Recombinant human STT3A protein Source: e coli.-derived, PGEX-4T Tag: GST Domain: 550-600 aa of BC020965 Sequence: THISRVGQAMASTEEKAYEIMRELDVSYVLVIFGGLTGYSSDDINKFLWMV 相同性解析による交差性が予測される生物種 |
| Full Name | STT3, subunit of the oligosaccharyltransferase complex, homolog A (S. cerevisiae) |
| Calculated molecular weight | 705 aa, 81 kDa |
| GenBank accession number | BC020965 |
| Gene Symbol | STT3A |
| Gene ID (NCBI) | 3703 |
| Conjugate | Unconjugated |
| Form | |
| Form | Liquid |
| Purification Method | Protein A purfication |
| UNIPROT ID | P46977 |
| Storage Buffer | PBS with 0.02% sodium azide and 50% glycerol{{ptg:BufferTemp}}7.3 |
| Storage Conditions | Store at -20°C. Stable for one year after shipment. Aliquoting is unnecessary for -20oC storage. |
Background Information
STT3A, also named as Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit STT3A, is a 705 amino acid prtein, which belongs to the STT3 family. STT3A is expressed at high levels in placenta, liver, muscle and pancreas, and at very low levels in brain, lung and kidney. STT3A is a catalytic subunit of the N-oligosaccharyl transferase (OST) complex which catalyzes the transfer of a high mannose oligosaccharide from a lipid-linked oligosaccharide donor to an asparagine residue within an Asn-X-Ser/Thr consensus motif in nascent polypeptide chains. N-glycosylation occurs cotranslationally and the complex associates with the Sec61 complex at the channel-forming translocon complex that mediates protein translocation across the endoplasmic reticulum (ER). SST3A seems to be involved in complex substrate specificity. STT3A is present in the majority of OST complexes and mediates cotranslational N-glycosylation of most sites on target proteins, while STT3B-containing complexes are required for efficient post-translational glycosylation and mediate glycosylation of sites that have been skipped by STT3A.