Product Information
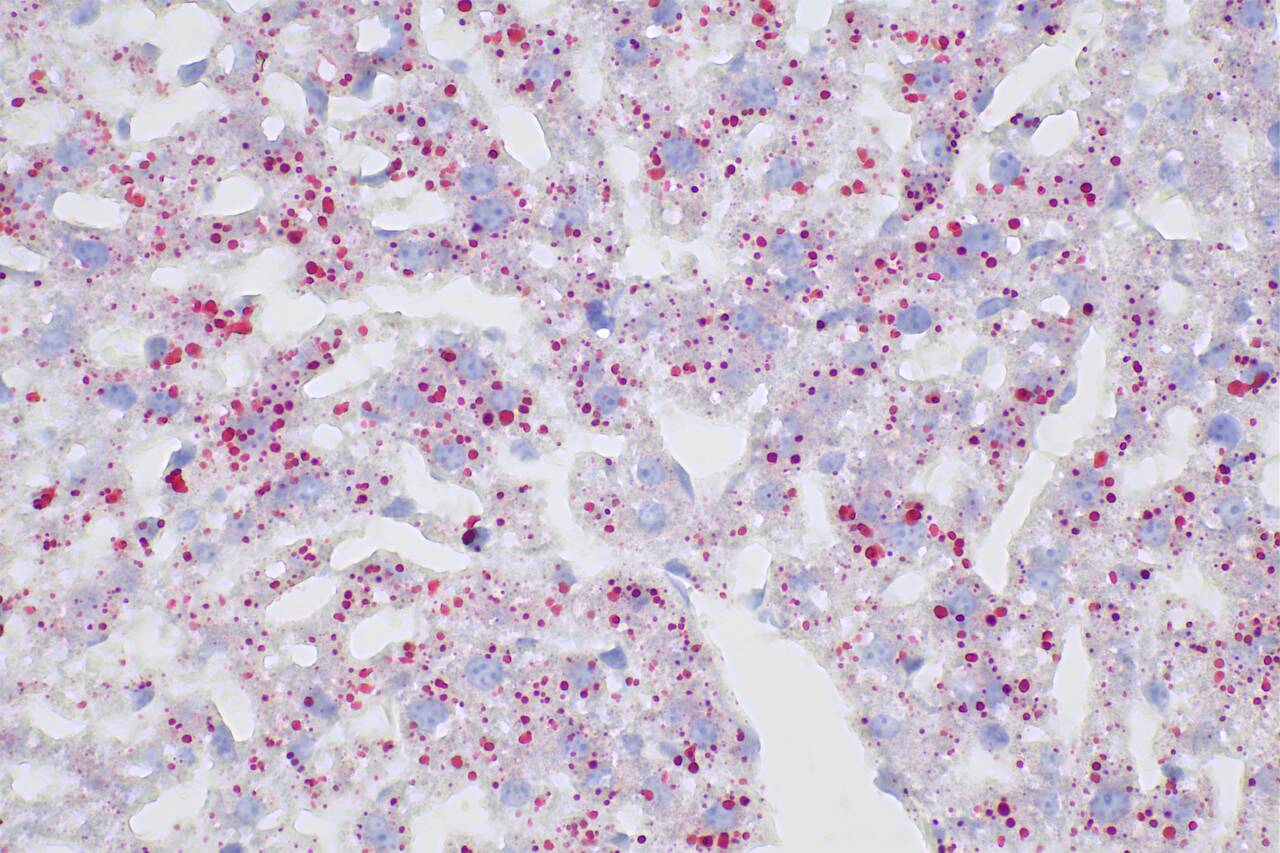
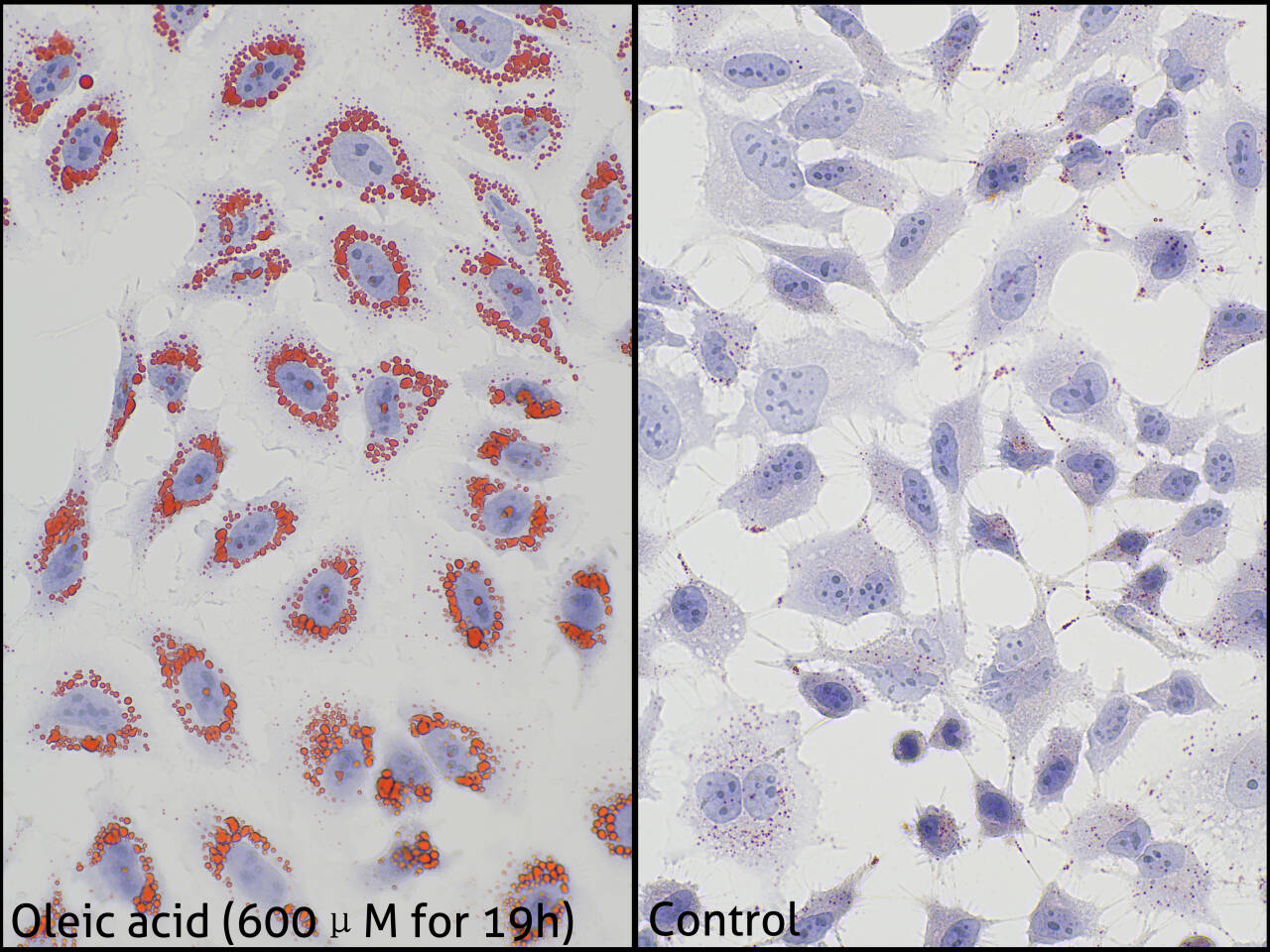
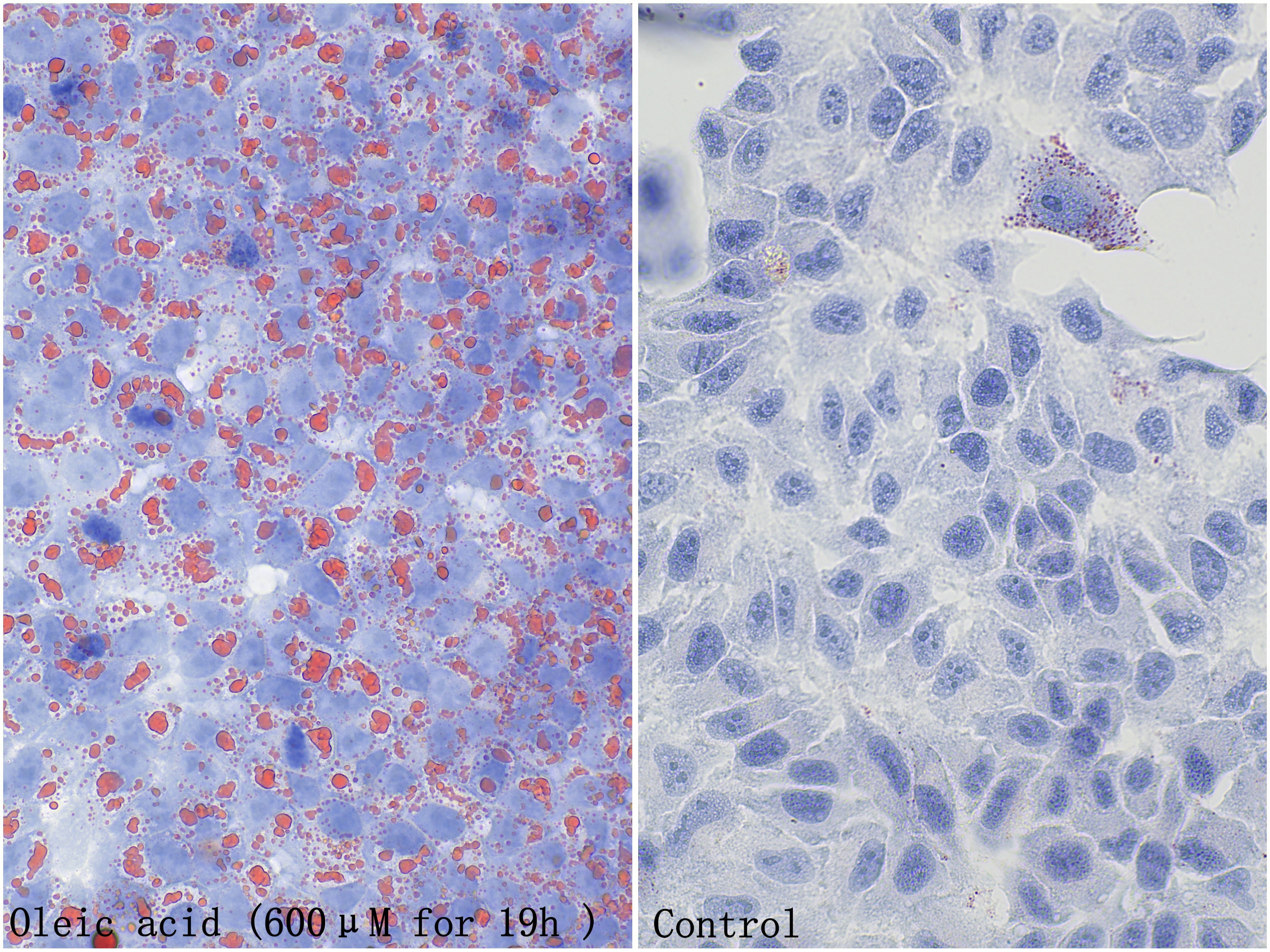
Oil Red O is a lipid-soluble diazo dye that specifically stains neutral lipids such as triglycerides in cells and tissues but has weaker staining effects on phospholipids and steroids. This product can be used in lipid-related studies, staining intracellular lipid droplets, triglycerides, and other neutral lipids in tissues red or orange red. It also contains hematoxylin, which can be used to counterstain cell nuclei, making them appear blue for observation. This product is suitable for staining frozen tissue and cell sections. Paraffin sections, however, are difficult to stain with this kit due to significant lipid loss during sample processing.
Components
This product contains sufficient materials to stain approximately 200 tissue sections or cell slides. Alternatively, it can also be used to stain approximately 400 wells in a 96-well plate. Note that the number of uses may vary depending on the size of the sections or the type of plate used. The main components are shown in the table below; please prepare any other reagents not listed.
Component | Specification |
Oil Red O Solution (Concentrated) | 12 mL |
Washing Buffer | 40 mL |
Mayer's Hematoxylin Solution | 20 mL |
Storage
Store at room temperature, away from light, for up to 1 year.
Usage
1. Preparation of Oil Red O Staining Working Solution:
a. Before the experiment, prepare the Oil Red O working solution according to the number of samples and the volume of working solution required for each sample, using the Oil Red O solution (concentrated) and ddH2O (self-prepared) in a 3:2 ratio. For example, if you need to prepare 10 mL of Oil Red O working solution, take 6 mL of Oil Red O solution (concentrated) and add 4 mL of ddH2O. Mix well to combine.
2. Oil Red O Staining:
a. Cell Samples
i. Slowly remove the cell culture medium from the slide or well and wash once with PBS. Note that the PBS used in this step must be self-prepared; do not use the washing buffer provided in the kit instead.
ii. Fix the cells with 4% paraformaldehyde fixative for 10 minutes, discard the fixative, and wash twice with PBS.
iii. Add an appropriate amount of the washing buffer provided in this kit to the cells and let stand for 20 seconds. Note that the volume of liquid should be enough to ensure complete coverage of all cells (approximately 50 uL/well for a 96-well plate, approximately 200 uL/well for a 24-well plate, approximately 300 uL/well for a 6-well plate, and approximately 100 uL/slide for a 1 cm diameter slide.
iv. Discard the washing buffer and add an appropriate amount of the prepared Oil Red O working solution to evenly cover the cells, and stain for 5-15 minutes.
v. Discard the Oil Red O working solution, wash the differentiated cells with washing buffer for 5-20 seconds, and then wash 3 times with PBS.
vi. Add the Mayer's Hematoxylin Solution provided in this kit and cover the cells for 2 minutes.
vii. Discard the hematoxylin staining solution, add PBS, soak for 5 minutes, and allow the cells to slowly turn blue.
viii. Observe under a microscope.
b. Frozen Tissue Sections
i. Remove the prepared frozen sections and place them on a slide rack to warm up for 5-10 minutes.
ii. Add an appropriate amount of washing buffer provided in this kit to completely cover the sections and let them stand for 20 seconds.
iii. Remove the washing solution and add an appropriate amount of the prepared Oil Red O working solution. Stain for 5-15 minutes.
iv. Remove the Oil Red O working solution, wash the differentiated tissue with washing buffer for 5-20 seconds, and rinse 3 times with PBS.
v. Add Mayer's Hematoxylin Solution, the hematoxylin staining solution provided in this kit and cover the tissue for 2 minutes.
vi. Immerse tissue slide in PBS for 5 minutes, allowing it to slowly turn blue, and rinse with ddH2O.
vii. Mount the slide with ddH2O or an aqueous mounting medium (Proteintech, catalog number PR30005) and observe under a microscope.
Cited in Article as
pk10040, Oil Red O staining kit, Proteintech, IL, USA
Documentation
| Datasheet |
|---|
| Oil Red O staining kit Datasheet |