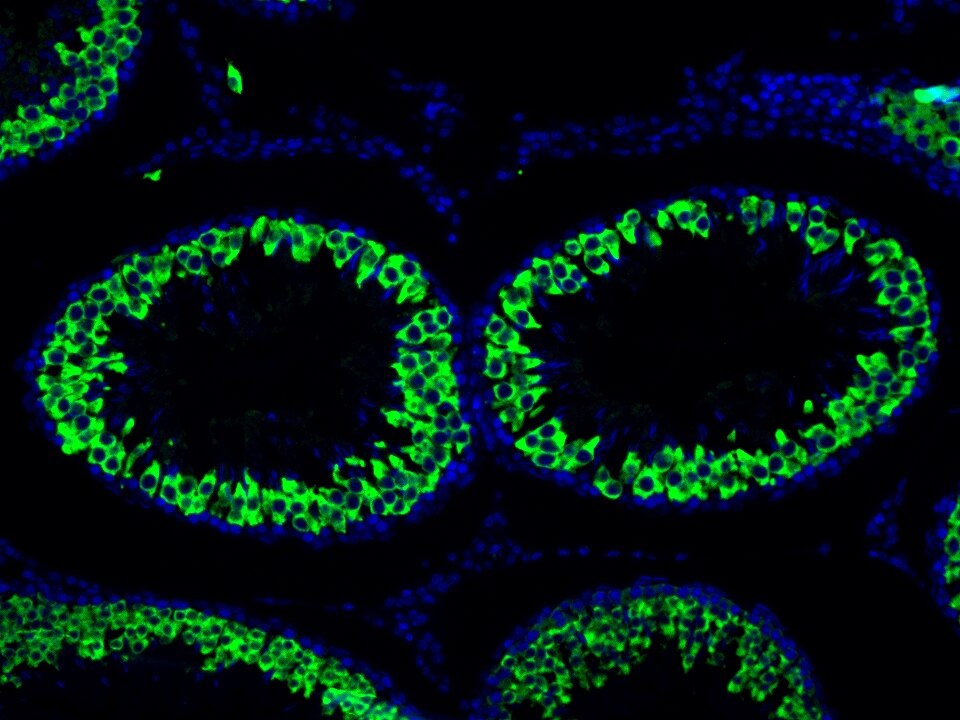
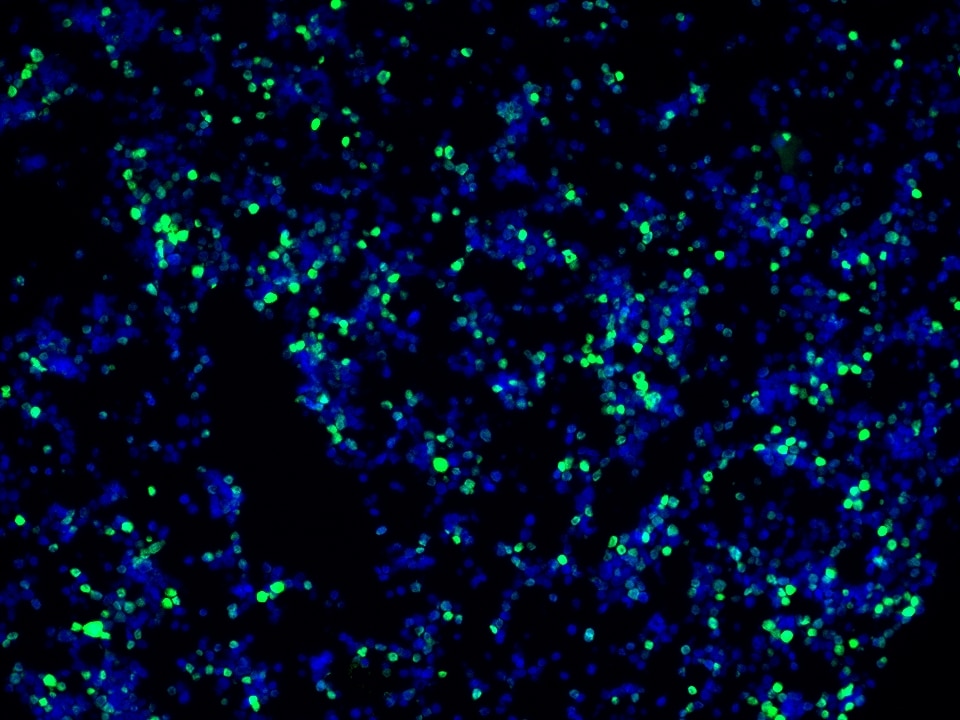
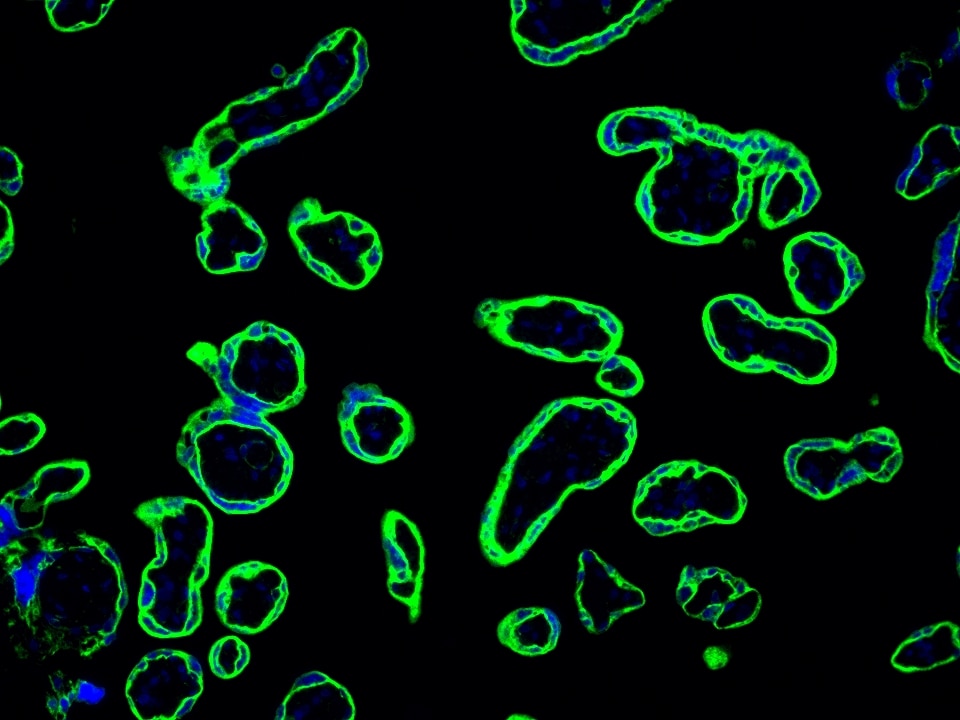
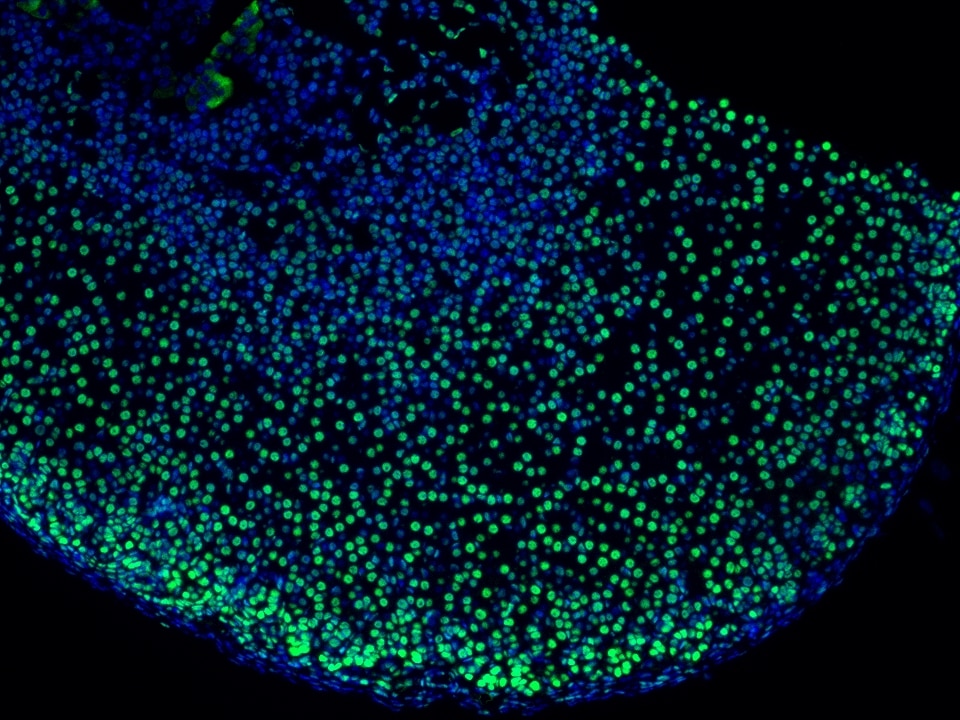
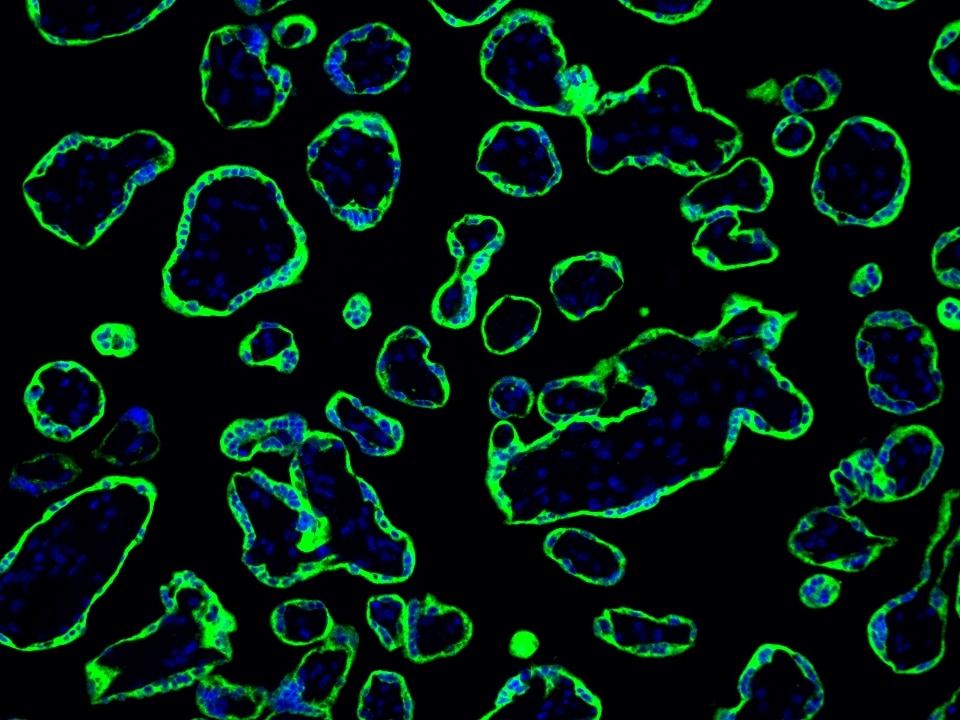
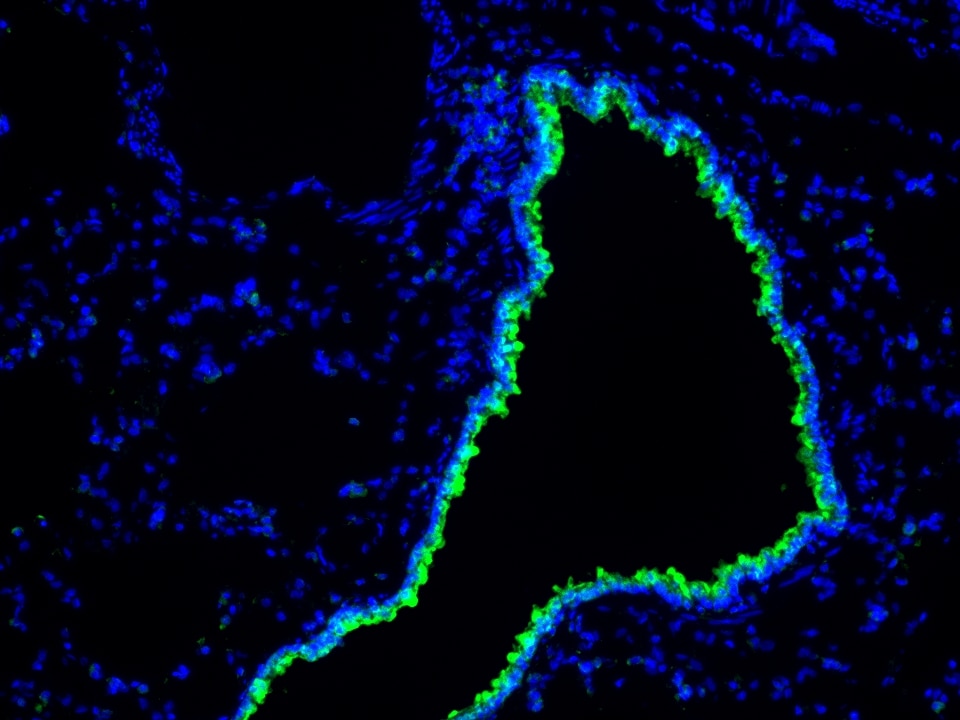
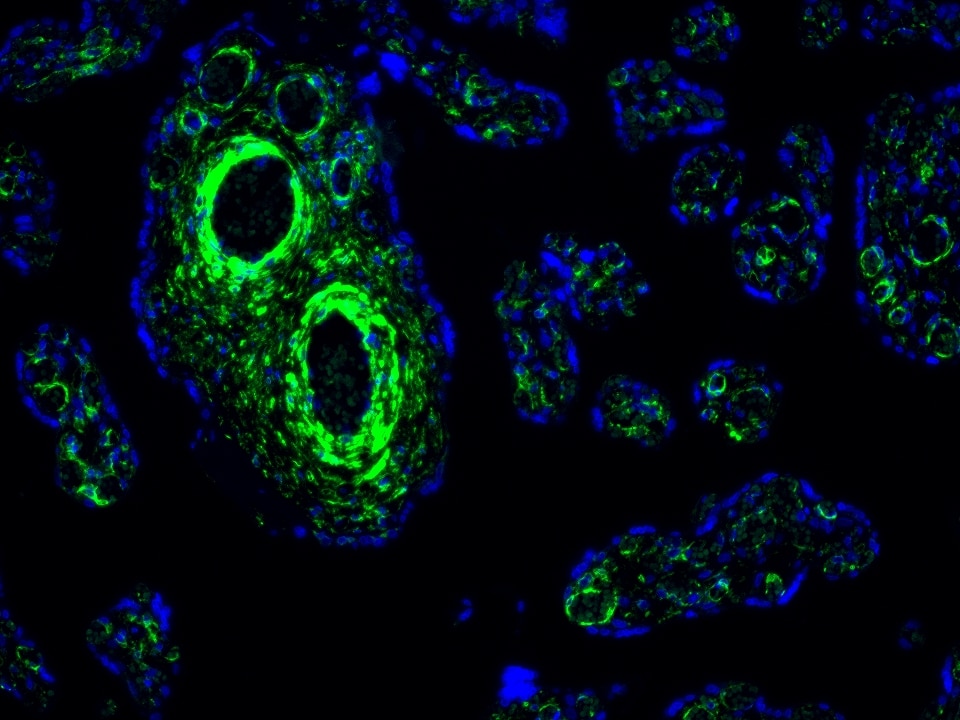
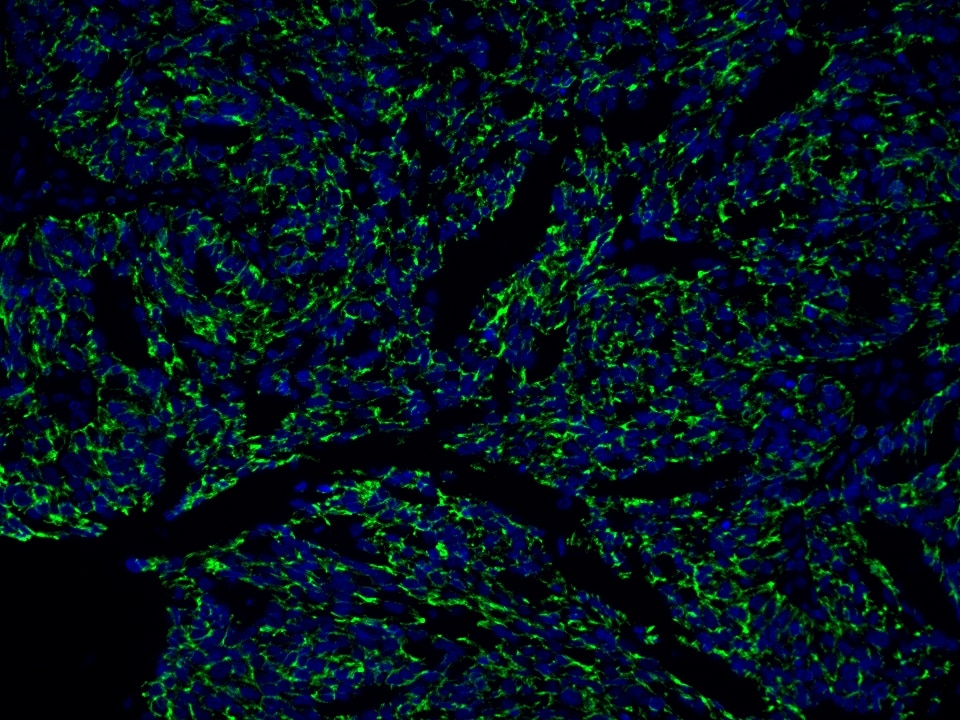
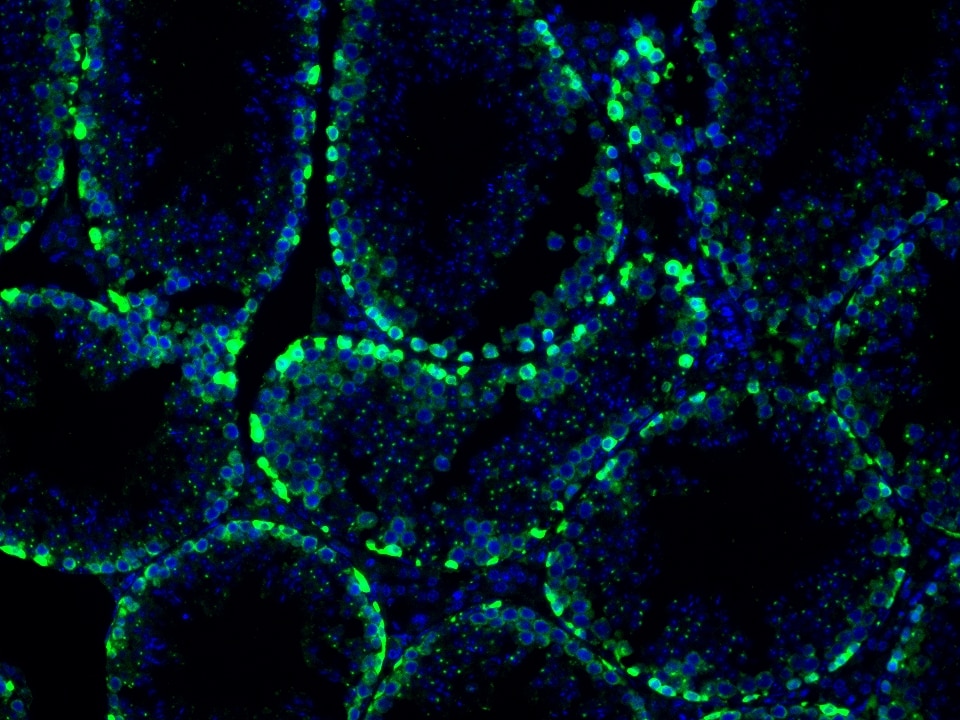
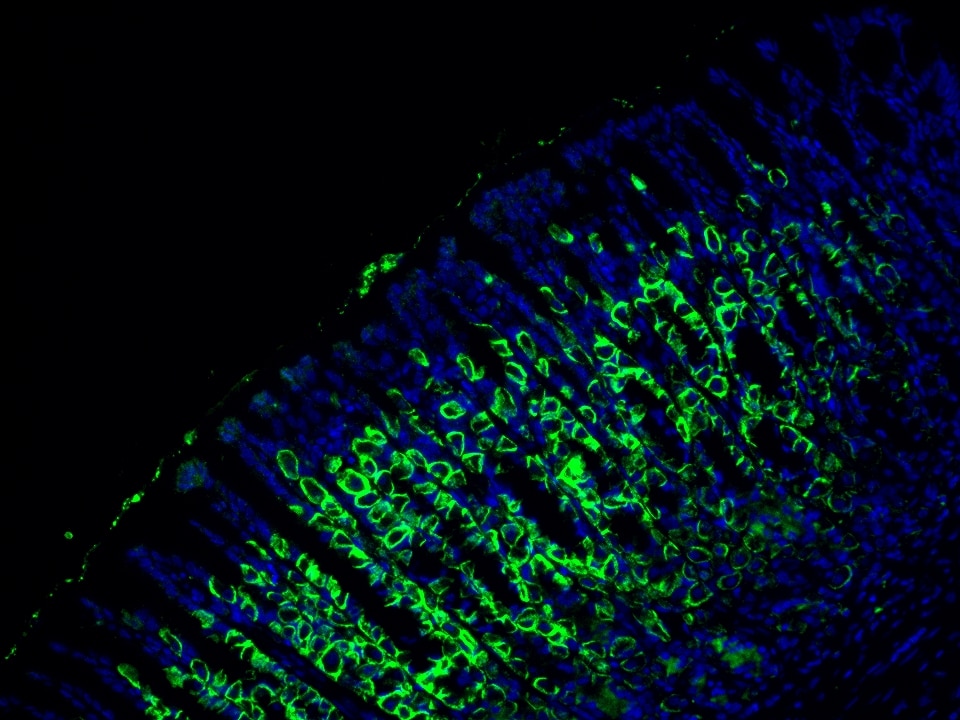
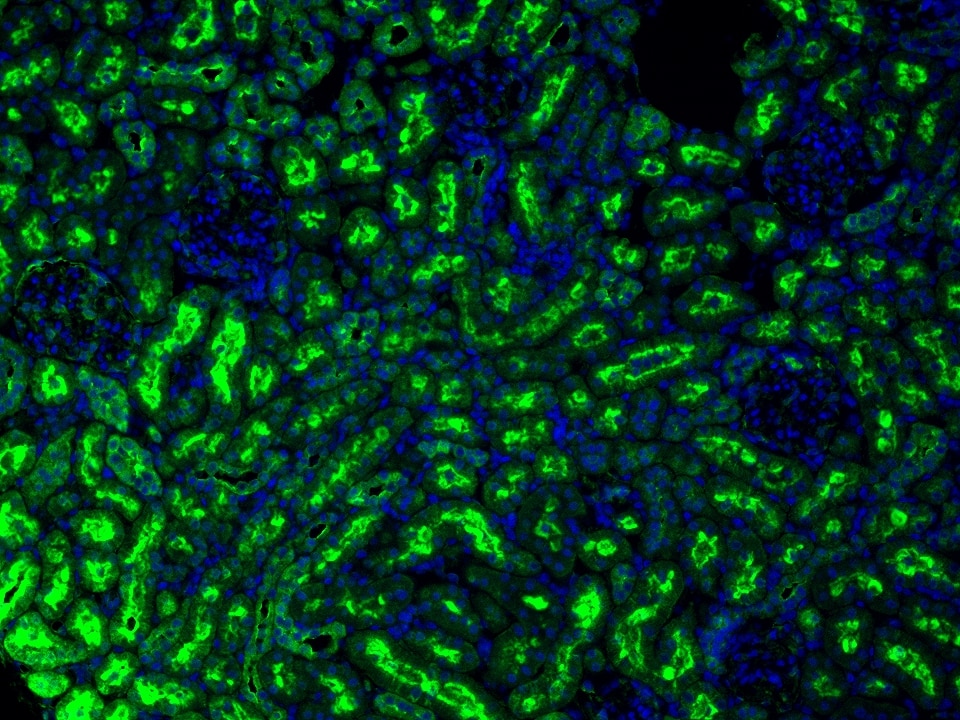
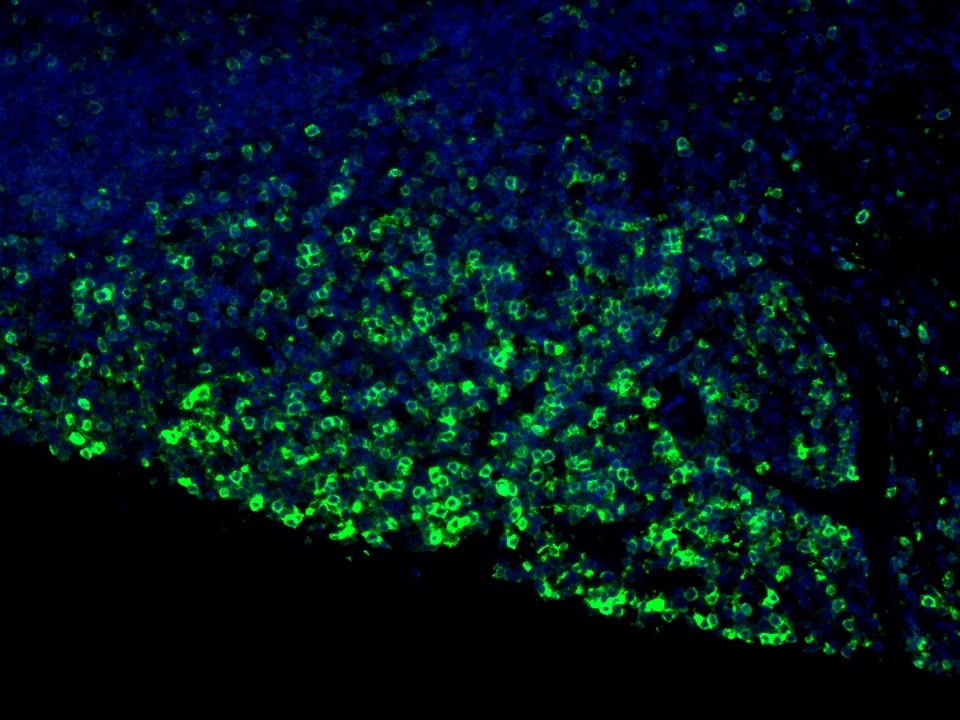
Product Information
TSA (Tyramide Signal Amplification) dyes are fluorescent labelling reagents based on tyramide signal amplification technology. Catalyzed by HRP, TSA dyes form covalent bonds with tyrosine residues on proteins near HRP and accumulate, thereby amplifying the signal. Compared to indirect detection using directly labeled fluorescent primary antibodies or fluorescent secondary antibodies, the TSA system can enhance the signal by several hundred-fold. Furthermore, the covalent bonds formed with the sample are stable and do not easily detach, making this technique useful for experiments such as multiplex immunofluorescence and in situ hybridization.
This product contains 100 uL CoraLite® Plus 488-TSA dye and 5 mL of amplification buffer, sufficient to stain approximately 50 tissue sections or cell slides. Note that the number of uses may vary slightly depending on the size of the slides. Please prepare or purchase other reagents required for your experiment.
The maximum absorption wavelength of the fluorescence signal of this product is 493 nm, and the maximum emission wavelength is 522 nm. Please use an appropriately configured imaging device for imaging.
Storage
Store at 2-8℃, valid for 12 months.
Protocols
Paraffin section staining
I. Dewaxing
1. Prepare tissue sections as standard. Mark the sections with a pen that is insoluble in xylene and ethanol and place them in a slide basket or staining rack (Always use a pen that is insoluble in xylene and ethanol; a graphite pencil will also work).
2. Soak the tissue sections in a xylene bath for 20 minutes, then repeat in another bath. Use fresh xylene both times.
3. Soak the tissue sections in 100% ethanol for 5 minutes, then repeat in another container. Use fresh ethanol both times.
4. Rehydrate the tissue sections by sequentially incubating with 95%, 80%, and 60% ethanol for 5 minutes:
5. Rince the tissue sections twice with fresh double-distilled water (ddH2O) for 1 minute each time.
II. Antigen retrieval
Perform antigen retrieval according to the method recommended by the primary antibody supplier. For example, if you are using a Proteintech primary antibody, pH 9.0 Tris-EDTA retrieval is recommended in most instances.
The specific method for Proteintech antibodies is as follows:
1. Heat the antigen retrieval solution on a hot plate until boiling, then reduce the heat ( Cover the beaker with tin foil to minimize evaporation).
2. Place the slice basket in the heated antigen retrieval solution and heat at 95-98 °C for 15-20 minutes.
3. Turn off the electric stove, remove the beaker from the stove and let it cool naturally to room temperature (about 35-40 minutes).
III. Inactivation and Blocking
1. Rinse the sections with ddH2O. Use a tissue pen to draw a circle around the tissue section on the slide. Rinse the sections with ddH2O and then rinse once with 1x TBST.
2. Inactivation (optional): Add 2 drops/100 uL Quenching Buffer (usually 3% H2O2) onto the tissue sections, ensuring that the liquid completely covers the tissue. Incubate the sections in a humidified chamber at room temperature for 10 minutes. After inactivation, wash the slides with 1x TBST.
In our experience, inactivation of endogenous peroxidase is not necessary in most cases and can even adversely affect staining. However, this step is widely documented in the literature and has been provided here for informational purposes only.
3. Blocking: Shake off the liquid on the slide. Add 2 drops/100 uL of blocking solution (such as 3% BSA) to the slide, covering the entire tissue section. Block the slide in a humidified chamber at room temperature for 30 minutes. After blocking, shake off the blocking solution.
IV. Primary Antibody Incubation
1. Dilute the primary antibody in an appropriate diluent. For initial experiments, it is recommended to test several dilutions of the primary antibody. Initially, you can start with a primary antibody concentration of 0.1-1 ug /mL or set up a gradient based on the immunohistochemical DAB staining results. Apply ~100 uL (the volume will depend on the size of the tissue) of the diluted primary antibody to the slide, covering the entire tissue.
2. Incubate at room temperature for 1 hour in a sealed humidified chamber (Be careful not to let the tissue sections dry out).
3. Rinse the primary antibody from the slides using a wash bottle. Then, immerse the slides in 1x TBST for 3 minutes. Repeat the wash step twice in fresh 1x TBST, shaking off any excess wash buffer.
V. Secondary Antibody Incubation
1. Add an appropriate amount of secondary antibody working solution to the slide to cover the entire tissue.
To obtain high-quality experimental results, it is recommended to use Proteintech 's recombinant secondary antibodies:
• RGAR011, Multi-rAb® Polymer HRP-Goat Anti-Rabbit Recombinant Secondary Antibody (H+L);
• RGAM011, Multi-rAb® Polymer HRP-Goat Anti-Mouse Recombinant Secondary Antibody (H+L);
2. Incubate at room temperature in a humidified chamber for 30 minutes (Be careful not to let the tissue sections dry out).
3. Rinse the secondary antibody from the slides using a wash bottle. Then, immerse the slides in 1x TBST for 3 minutes. Repeat the wash step twice in fresh 1x TBST, shaking off any excess wash buffer.
4. Bring the TSA dye and TSA diluent to room temperature in a dark place away from the light. Prepare the TSA working solution based on the desired volume. Mix CoraLite® Plus 488-TSA dye and amplification buffer at a ratio of 1:50.
VI. TSA Dye Incubation
1. After washing away the unbound secondary antibody, add TSA working solution to the slide, ensuring that the entire tissue is covered.
2. Incubate in a sealed humidified chamber at room temperature for 10 minutes in the dark.
3. Rinse the TSA stain on the slides with ddH2O and rinse the sections twice with fresh ddH2O.
If you need to perform multiple staining, proceed to section VII (Multiple Staining). If you want to stain only one color, proceed to section VIII (Nuclear Staining).
VII. Multiple staining
1. Perform antigen retrieval after one round of staining: Use Tris-EDTA pH 9.0 antigen retrieval solution and perform the same steps as in section II (Antigen Retrieval). Be careful not to allow the liquid to boil and create bubbles, as this can cause the slide to peel off.
2. After antigen retrieval is complete, repeat the steps in sections III-VI to complete the second round of antibody staining.
3. Repeat steps in sections II-VI to complete multiple rounds of antigen retrieval, blocking, primary antibody, secondary antibody, and TSA staining. If you need to pause the experiment, it is recommended that you soak the tissue sections in fresh ddH2O after TSA staining and store them at 4°C in the dark.
VIII. Nuclear Staining
1. After TSA staining, rinse the slides with fresh ddH2O to remove the TSA stain. Rinse the sections twice with fresh ddH2O. Rinse the sections once with 1x TBST. Add ~100 uL (the volume depends on the size of the tissue) of DAPI stain to the slide, covering the entire tissue.
2. Incubate in a sealed humidified chamber at room temperature in the dark for 10 minutes.
3. Rinse the DAPI stain on the slide with ddH2O and rinse the sections twice with fresh ddH2O each time.
IX. Sealing and Observation of Results
1. Mounting: Allow the sections to air-dry in a dark place. Add one drop of mounting medium to the tissue section. Carefully place a coverslip over the tissue and mounting medium, ensuring that the tissue is completely covered by the mounting medium to avoid bubbles.
2. Observation: Observe and analyze the staining results using a fluorescence microscope or other imaging equipment.
Frozen section staining
I. Take out the prepared frozen sections and return them to room temperature.
II. Antigen retrieval (optional)
Determine whether antigen retrieval is necessary based on the primary antibody supplier's recommendations. This step can be skipped for primary antibodies that do not require retrieval. The specific retrieval method should be based on the primary antibody supplier's recommendations.
III. Inactivation and Blocking
1. Rinse the sections with ddH2O. Use a tissue pen to draw a circle around the tissue section on the slide. Rinse the sections with ddH2O and then rinse once with 1x TBST.
2. Blocking: Shake off the liquid on the slide and add 2 drops/100 uL of blocking solution (e.g., 3% BSA) to the slide, covering the entire tissue section. Block in a humidified chamber at room temperature for 30 minutes. After blocking, shake the blocking solution off the slide.
IV. Primary Antibody Incubation
1. Dilute the primary antibody in an appropriate diluent. For initial experiments, it is recommended to test several dilutions of the primary antibody. Initially, you can start with a primary antibody concentration of 0.1-1 ug /mL or set up a gradient based on the immunohistochemical DAB staining results. Apply ~100 uL (the volume will depend on the size of the tissue) of the diluted primary antibody to the slide, covering the entire tissue.
2. Incubate at room temperature for 1 hour in a sealed humidified chamber (Be careful not to let the tissue sections dry out).
3. Rinse the primary antibody from the slides using a wash bottle. Then, immerse the slides in 1x TBST for 3 minutes. Repeat the wash step twice in fresh 1x TBST, shaking off any excess wash buffer.
V. Secondary Antibody Incubation
1. Add an appropriate amount of secondary antibody working solution to the slide to cover the entire tissue.
For best results, it is recommended to use Proteintech's recombinant secondary antibodies:
• RGAR011, Multi-rAb® Polymer HRP-Goat Anti-Rabbit Recombinant Secondary Antibody (H+L);
• RGAM011, Multi-rAb® Polymer HRP-Goat Anti-Mouse Recombinant Secondary Antibody (H+L);
2. Incubate at room temperature in a humidified chamber for 30 minutes (Be careful not to let the tissue sections dry out).
3. Rinse the secondary antibody from the slides using a wash bottle. Then, immerse the slides in 1x TBST for 3 minutes. Repeat the wash step twice in fresh 1x TBST, shaking off any excess wash buffer.
4. Bring the TSA dye and TSA diluent to room temperature in a dark place away from the light. Prepare the TSA working solution based on the desired volume. Mix CoraLite® Plus 488-TSA dye and amplification buffer at a ratio of 1:50.
VI. TSA Dye Incubation
1. After washing away the unbound secondary antibody, add TSA working solution to the slide, ensuring that the entire tissue is covered.
2. Incubate in a sealed humidified chamber at room temperature for 10 minutes in the dark.
3. Rinse the TSA stain on the slides with ddH2O and rinse the sections twice more with fresh ddH2O each time.
If you need to perform multiple staining, proceed to section VII. If you want to stain only one color, skip to section VII.
VII. Multiple staining
1. After one round of staining, remove the primary and secondary antibodies from the sections with Stripping Buffer. The stripping could be performed at 65℃ using the following recipe: 2% SDS + 0.8% Beta-Mercaptoethanol in 62.5mM Tris (pH 6.8). Other methods from publications may also be applicable.
2. Repeat the steps in sections III-VI to complete the second round of antibody staining.
3. Repeat the steps in sections III-VII to complete multiple rounds of regeneration, blocking, primary antibody, secondary antibody, and TSA staining. If you need to pause the experiment, it is recommended to soak the slides in fresh ddH2O after TSA staining and store it at 4°C in the dark.
VIII. Nuclear Staining
1. After TSA staining, rinse the slides with fresh ddH2O to remove the TSA stain. Rinse the sections twice with fresh ddH2O. Rinse the sections once with 1x TBST. Add ~100 uL (the volume depends on the size of the tissue) of DAPI stain to the slide, covering the entire tissue.
2. Incubate in a sealed humidified chamber at room temperature in the dark for 10 minutes.
3. Rinse the DAPI stain on the slide with ddH2O and rinse the sections twice with fresh ddH2O.
IX. Sealing and Observation of Results
1. Mounting: Allow the sections to air dry in a dark place. Add one drop of mounting medium to the tissue section. Carefully place a coverslip over the tissue and mounting medium, ensuring that the tissue is completely covered by the mounting medium to avoid bubbles.
2. Observation: Observe and analyze the staining results using a fluorescence microscope or other imaging equipment.
Cell sample staining
I. Fix Cells
1. Discard the cell culture medium and add enough 1× PBS to the cell slide or culture plate (~100 uL/slide,~ 50 uL/well of 96-well plate) to wash the cells for 3 minutes each time, drain and discard the wash solution, and repeat twice.
2. Add enough fixative to the cells and fix for 15 minutes at room temperature. Use the appropriate fixative according to the primary antibody manufacturer's recommendations. When performing multiple rounds of staining, ensure that the fixative is compatible with all primary antibodies.
3. Discard the fixative solution and wash the cells with sufficient 1× PBS for 3 minutes each time, drain and discard the wash solution, and repeat twice.
II. Permeabilization and Blocking
1. Add (0.2% Triton X-100) to the cells and permeabilize for 5 minutes at room temperature. Discard the permeabilization solution and wash the cells twice with sufficient 1× PBS for 3 minutes. Drain and discard the solution. Repeat this cycle twice.
2. Add sufficient blocking solution (3% BSA) to the cells. Block at room temperature for 30-60 minutes. After blocking, drain and discard the blocking solution.
III. Primary antibody incubation
1. Dilute the primary antibody in an appropriate diluent. For initial experiments, it is recommended to test several dilutions of the primary antibody. During this trial, you can set a gradient around a primary antibody concentration of 0.1-1 ug /mL or based on the immunohistochemical DAB staining conditions for this primary antibody.
2. Add sufficient primary antibody working solution to the cells and incubate at 37°C for 1 hour or at room temperature for 2 hours.
3. Discard the primary antibody and wash the cells with sufficient 1× PBS for 3 minutes. Drain the wash solution and repeat twice.
IV. Secondary Antibody Incubation
1. Add an appropriate amount of secondary antibody working solution to the cells, ensuring that all cell areas are covered. Incubate at room temperature for 30 minutes.
For the best experimental results, it is recommended to use Proteintech 's recombinant secondary antibodies:
• RGAR011, Multi-rAb® Polymer HRP-Goat Anti-Rabbit Recombinant Secondary Antibody (H+L);
• RGAM011, Multi-rAb® Polymer HRP-Goat Anti-Mouse Recombinant Secondary Antibody (H+L);
2. Discard the secondary antibody and wash the cells with ample 1× PBS for 3 minutes. Drain and repeat twice.
3. Bring the TSA dye and TSA diluent to room temperature in a dark place away from the light. Prepare the TSA working solution based on the desired volume. Mix CoraLite® Plus 488-TSA dye and amplification buffer at a ratio of 1:50.
V. TSA Dye Incubation
1. After washing with the secondary antibody, add TSA working solution dropwise to the cells, ensuring that all cell areas are covered. Incubate at room temperature in the dark for 10 minutes.
2. Discard the dye and wash the cells with ample 1× PBS for 3 minutes. Drain and dry the wash solution. Repeat this step twice.
3. If you need to perform multiple rounds of staining, proceed to section VI. If you want to stain only one color, proceed to section VII.
VI. Multiplexed staining
1. After one round of staining, use Stripping Buffer to remove the primary and secondary antibodies from the cells. The stripping could be performed at 65℃ using the following recipe: 2% SDS + 0.8% Beta-Mercaptoethanol in 62.5mM Tris (pH 6.8). Other methods from publications may also be applicable.
2. Repeat the steps in sections III-V to complete the second round of antibody staining.
3. Repeat the steps III-VI to complete multiple rounds of regeneration, blocking, primary antibody, secondary antibody, and TSA staining. If you need to pause the experiment, it is recommended that you soak the cell sample in fresh PBS after TSA staining and store it at 4°C, protected from light.
VII. Nuclear Staining
1. Add appropriate amount of DAPI dye to the cells and incubate at room temperature in the dark for 10 minutes.
2. Discard the DAPI and wash the cells with ample 1× PBS for 3 minutes each time. Discard and drain the wash solution. Repeat twice. Depending on the image mode compatible with your imaging device, choose to observe after sealing the slides or add PBS to the cells and observe directly.
VIII. Results and Observation
Result observation: Observe and analyze the staining results using a fluorescence microscope or other imaging equipment.
Precautions
1. If performing multiplexed staining, it is generally recommended to stain low-abundance targets or weak antibodies first. For example, when using Proteintech TSA dyes, the recommended color matching order is: 555-488-594-647. Also, since CoraLite®Plus 555-TSA and CoraLite®Plus 594-TSA have similar wavelengths and are prone to bleed-through, it is recommended that CoraLite®Plus 555-TSA and CoraLite®Plus 594-TSA used with weakly positive primary antibodies.
2. If performing multiplexed staining, it is recommended to first perform single staining to explore the optimal conditions for different targets before performing multiple rounds of staining to reduce unnecessary waste and facilitate analysis of results.
3. During the operation, when adding liquid to the sample at any step, ensure that there are no bubbles in the liquid, otherwise abnormal staining such as plaques will form. When adding liquid, be careful not to scratch the tissue.
4. Pay attention to the tissue sections or cell samples during the experiment and prevent them from drying out.
Cited in Article as
pr30022, CoraLite®Plus 488-Tyramide Reagent, Proteintech, IL, USA
Documentation
| SDS |
|---|
| CoraLite®Plus 488-Tyramide Reagent |
| Protocol |
|---|
| Protocol-PR30022 |
| Datasheet |
|---|
| CoraLite®Plus 488-Tyramide Reagent Datasheet |