Human/Mouse/Rat SNAP25 ELISA Kit
Cat no : KE00031
Synonyms
RIC 4, RIC4, SEC9, SNAP, SNAP 25, SNAP25, SUP, Super protein
Validation Data Gallery
Product Information
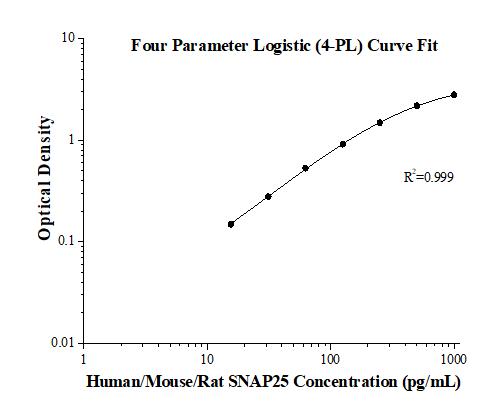
KE00031 is a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (Sandwich ELISA). The SNAP25 ELISA kit is to be used to detect and quantify protein levels of endogenous SNAP25. The assay recognizes human/mouse/rat SNAP25. An antibody specific for SNAP25 has been pre-coated onto the microwells. The SNAP25 protein in samples is captured by the coated antibody after incubation. Following extensive washing, another antibody specific for SNAP25 is added to detect the captured SNAP25 protein. For signal development, horseradish peroxidase (HRP)-conjugated antibody is added, followed by Tetramethyl-benzidine (TMB) reagent. Solution containing sulfuric acid is used to stop color development and the color intensity which is proportional to the quantity of bound protein is measurable at 450 nm with the correction wavelength set at 630 nm.
| Product name | Human/Mouse/Rat SNAP25 ELISA Kit |
| Tests | 1 X 96 well plate |
| Sample type | Tissue lysate |
| Assay type | Sandwich |
| Sensitivity | 6.0 pg/mL |
| Range | 15.6-1000 pg/mL |
| Reactivity | Human/Mouse/Rat |
| Tested applications | Sandwich ELISA |
| Gene ID (NCBI) | 6616 |
Recovery
| Sample Type | Average | Range |
|---|---|---|
| Tissue lysate | 97% | 81%-119% |
IntraAssay
| Sample | n | mean ( pg/mL) | SD | CV% |
|---|---|---|---|---|
| 1 | 20 | 36.9 | 1.1 | 2.9 |
| 2 | 20 | 334.6 | 11.2 | 3.3 |
| 3 | 20 | 604.7 | 27.9 | 4.6 |
InterAssay
| Sample | n | mean ( pg/mL) | SD | CV% |
|---|---|---|---|---|
| 1 | 24 | 47.1 | 2.9 | 6.0 |
| 2 | 24 | 439.0 | 30.3 | 6.9 |
| 3 | 24 | 841.1 | 67.0 | 8.0 |
Background Information
Synaptic vesicle membrane docking and fusion is mediated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex which is located on the vesicle membrane (v-SNAREs) and the target membrane (t-SNAREs). Other than VAMP2 and STX1A, SNAP25 is another key component of SNARE core complex, and is known to be involved in regulating neurotransmitter release. Its palmitoylation domain is located in the middle of the molecule that contains four cysteine residues and mutation of the cysteines abolishes palmitoylation and its membrane binding activity. As an important presynaptic plasma membrane protein, SNAP25 has been linked to memory and learning by its effect on long term potentiation in the hippocampus, thus playing a critical role in the synaptic function of specific neuronal systems. It has been reported that SNAP25 levels were were significantly decreased in brain tissue homogenates of patients with later stages of Alzheimer's disease compared with the controls, while the levels of SNAP25 were significantly increased in cerebrospinal fluid (CSF) of the group with an Alzheimer's disease biomarker profile than in the group with a control biomarker profile.
Properties
| Storage Instructions | All the reagents are stored at 2-8℃ for 6 months or -20℃ for 12 months. Refer to the protocol for further storage instructions. |
| Synonyms | RIC 4, RIC4, SEC9, SNAP, SNAP 25, SNAP25, SUP, Super protein |
Publications
| Species | Sample Type | Title |
|---|---|---|
Alzheimers Dement Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. | ||
Eur J Neurosci Associations between cerebrospinal fluid markers and cognition in aging and dementia: A systematic review. | ||
BMC Med Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer's disease |