Key considerations for raw material selection in cell therapy manufacturing
Learn more about regulatory requirements and best practices in sourcing raw materials for cell based therapy manufacturing
Introduction:
Cell therapy has emerged as a promising field, offering ground-breaking treatment options for a wide range of diseases and conditions. One crucial factor that ensures the safety and effectiveness of cell therapies is the careful selection of GMP (Good Manufacturing Practice) grade raw materials. This blog post will explore the critical considerations that researchers and manufacturers must take into account when selecting raw materials for cell therapy applications.
Safety and Quality:
Ensuring the safety and quality of raw materials is paramount in cell therapy manufacturing; both criteria are significantly influenced by material source and purityIt is essential to source materials that are free from impurities that could compromise the quality and safety of the final cell therapy product. In addition, the consideration of material source is vital as the use of animal-derived components in cell therapy raw materials can introduce risks such as contamination, transmission of infectious agents, and potential immune reactions in patients. As a result, there is a growing emphasis on sourcing raw materials that are devoid of any animal-derived components. This ensures a safer and more controlled finished product reducing the likelihood of adverse events and improving the overall quality and acceptability of cell therapy.
Regulatory Compliance:
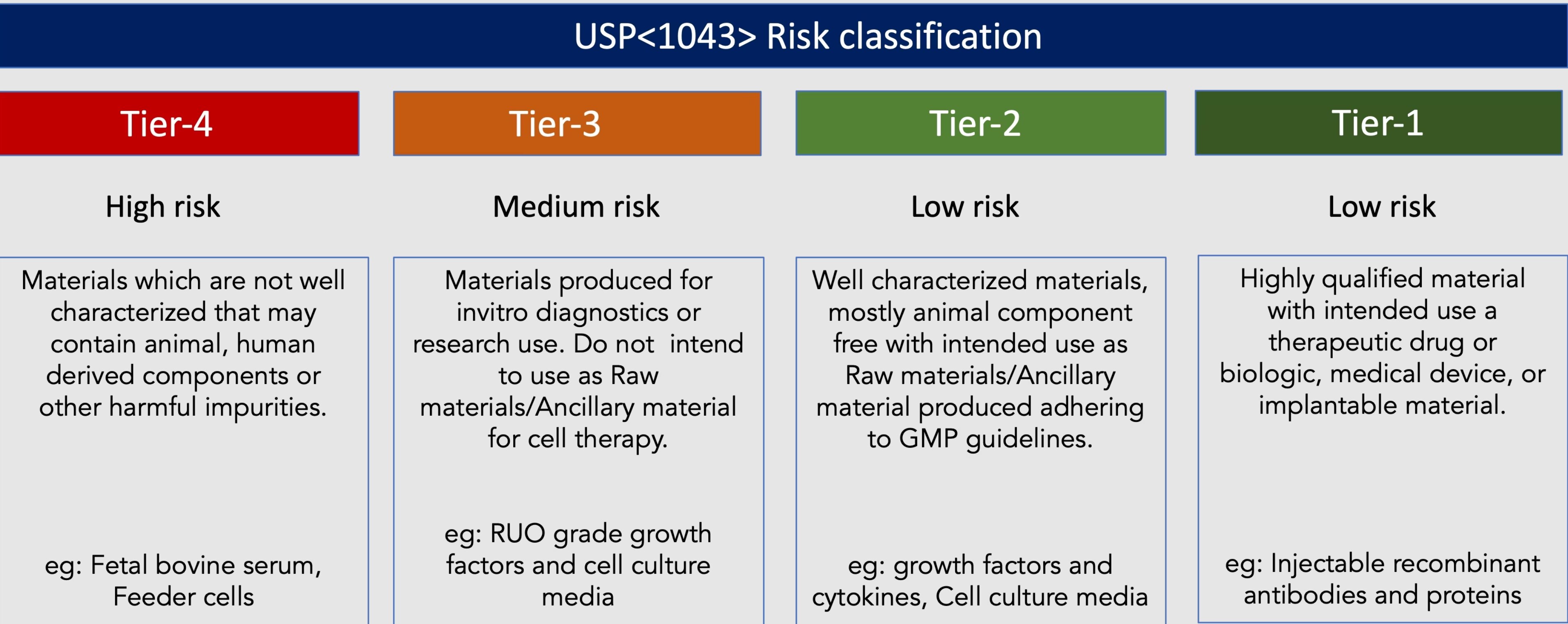
Adhering to regulatory requirements is of utmost importance in cell therapy development and commercialization. The selected raw materials should align with applicable regulatory guidelines and standards set forth by regulatory authorities. Products developed under specific quality management systems like ISO13485 should be preferred as the requirements with global regulatory guidelines, ensuring organizations meet the necessary standards for safety, quality, and compliance. This is achieved by meeting the standard's minimum requirements, including robust documentation, traceability, and validation of raw materials; all of which are essential to demonstrate compliance during regulatory inspections and approval processes. Selecting GMP-grade raw materials helps manufacturers meet the necessary documentation, testing, and quality control standards, positioning them favourably during regulatory inspections and assessments. Agencies like United States Pharmacopeia (USP) recommend guidelines to qualify raw materials for cell and gene therapies in chapter <1043> and <92>.
USP Chapter <1043> outlines the principles and recommendations for selecting, qualifying, and controlling ancillary materials, including raw materials, used in the production of cell, gene, and tissue-engineered products. It emphasizes the need for comprehensive risk assessments, characterization, and qualification of these materials to ensure their suitability and compatibility with cell therapy processes.
Functionality and Compatibility:
Raw materials used in cell therapy must possess the necessary functionality to facilitate desired cellular behavior. This includes cell proliferation, differentiation, and survival. It is important to select raw materials that are compatible with the specific cell types and intended therapeutic applications. Compatibility also extends to the formulation of culture media, supplements, and biomaterials to create an optimal environment for cell growth, viability, and functionality. Presence of unstable growth factors in cell culture media can lead to unintended differentiation of cells.
|
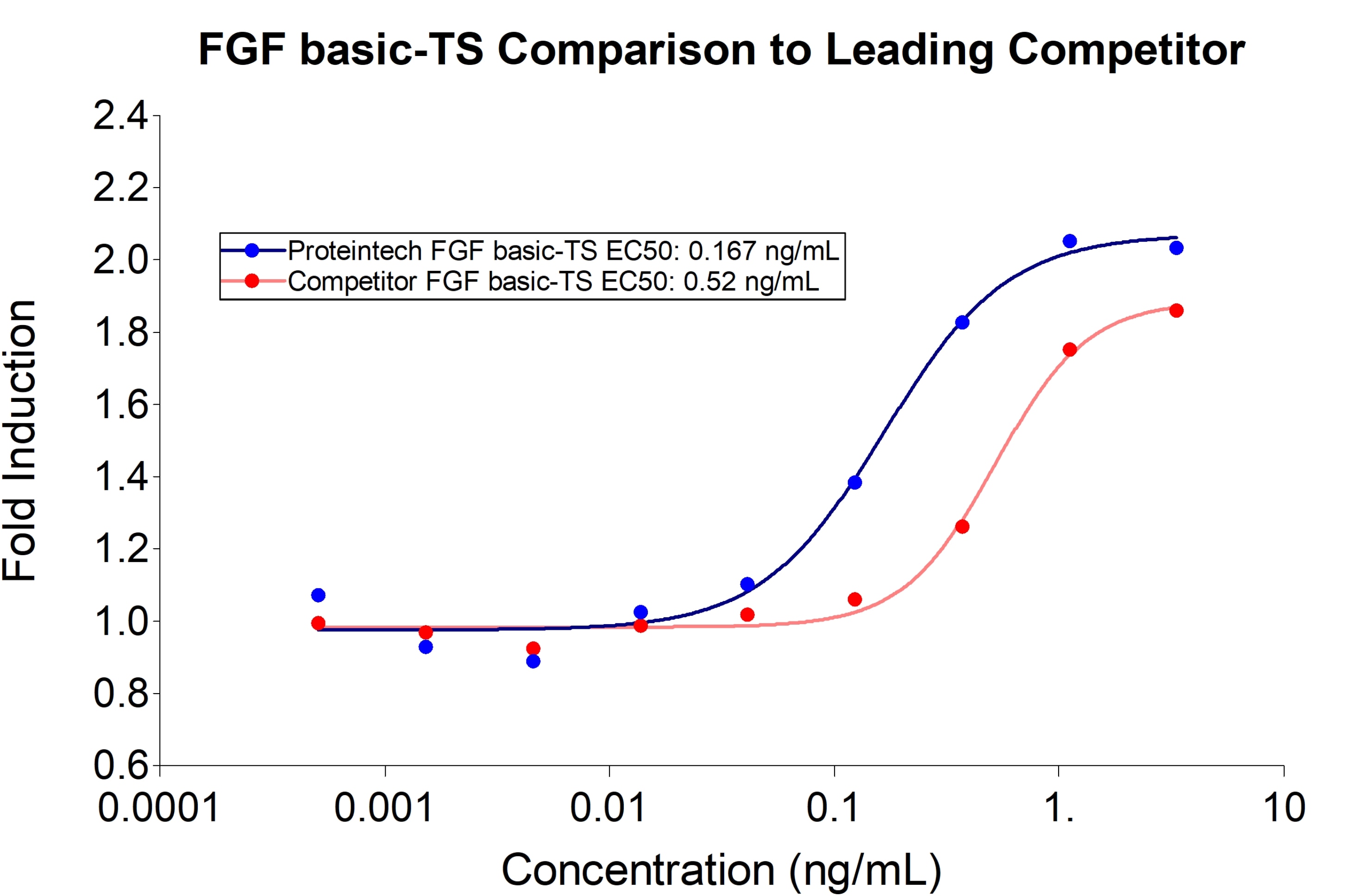
Proteintech FGF basic-TS (HZ-1285) demonstrates greater induction of proliferation and 3-fold lower EC50 compared to leading competitors. Recombinant human FGFbasic-TS stimulates dose-dependent proliferation of the HDFa human primary fibroblast cell line. |
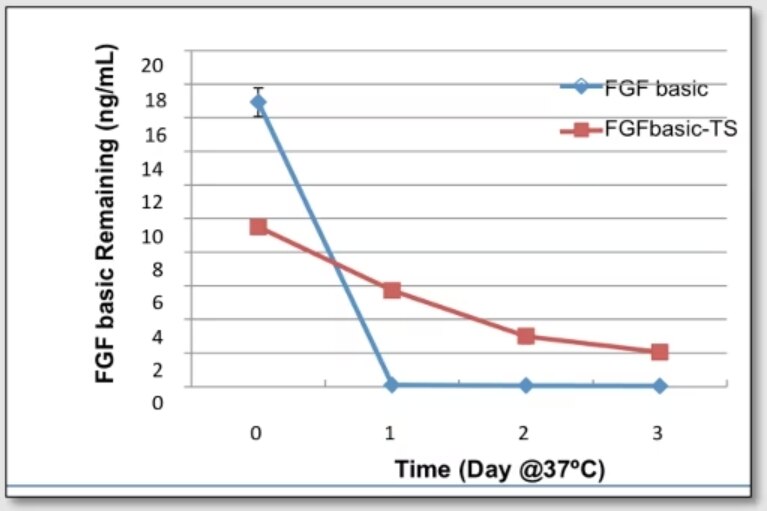
Proteintech’s FGF basic-TS (HZ-1285) in xeno free cell culture media is stable up to 3 days compared to similar products which degrades in one day.
|
Quality and Consistency:
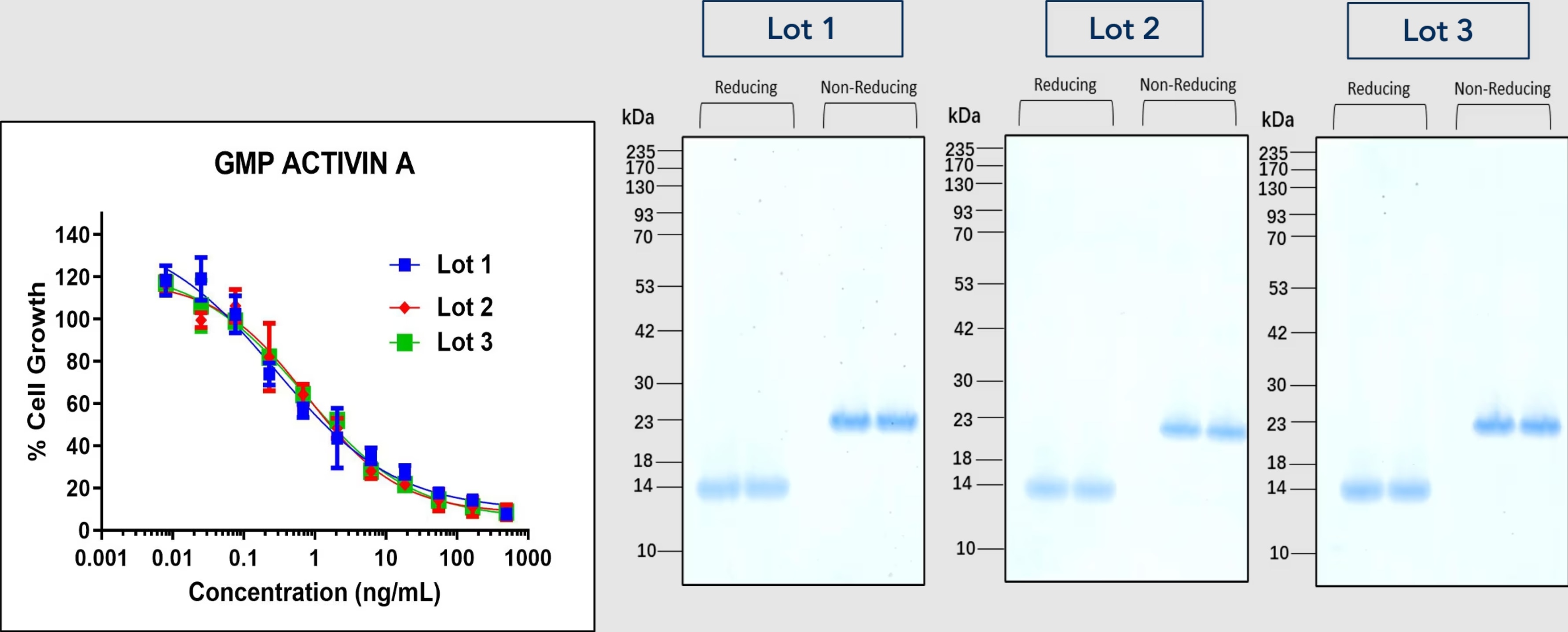
Consistency and reproducibility are paramount in cell therapy manufacturing. The raw material must be consistent between batches to ensure reliable and predictable results. Manufacturers should prefer to source raw materials from reliable suppliers with established quality control processes to ensure consistent product characteristics and performance. Maintaining consistency in raw material quality is important for study comparability, regulatory compliance, and successful transition from preclinical to clinical applications.
GMP-quality raw materials ensure batch-to-batch consistency, ensuring predictable behavior and therapeutic effect of manufactured cell therapies. Such consistency allows the results of different studies to be reliably compared and facilitate the evaluation of safety and efficacy in preclinical and clinical studies.
Scalability and Cost-effectiveness:
It is important to consider scalability and cost-effectiveness when choosing raw materials for the production of cell therapy. As cell therapy is rapidly progressing towards allogenic, raw materials must be available in sufficient quantities to support large-scale production without compromising quality. Manufacturers must evaluate the cost-effectiveness of raw materials to ensure commercial viability while maintaining high quality standards. Collaboration with suppliers and strategic sourcing can help optimize scalability and cost-effectiveness in raw material selection.
Raw material related responsibilities; Supplier Vs User
A new ISO standard ISO-20399 recommends user and supplier responsibilities when sourcing raw material for manufacturing cell based therapies. It notes that the cooperation and transparency between the raw material user and supplier play a crucial role. They collaborate to determine numerous responsibilities jointly, leveraging their combined efforts for mutual benefit. This supplier-user dynamic significantly contributes to the success of various activities. Without such a relationship, the user faces potential risks, like a lack of technical support from the supplier. It's important to emphasize that the responsibility for these activities is assessed on an individual basis.
ISO-20399 Recommendations of responsibilities and responsible parties
Activity |
Supplier |
User |
|
Provide documented evidence that the raw material/ancillary material is safe with respect to source-relevant animal diseases (e.g. BSE/TSE) |
✔ |
|
|
Prepare and submit a master file for raw material/ancillary material, if applicable |
✔ |
|
|
Assess the stability of the raw material/ancillary material |
✔ |
|
|
Inform the raw material/ancillary material user of any changes that will very likely or with certainty impact the raw material/ancillary material (e.g. under a quality agreement) |
✔ |
|
|
Conduct an assessment of the raw material/ancillary material container closure system |
✔ |
|
|
Provide a CoA, CoO and SDS for the raw material/ancillary material. |
✔ |
|
|
Conduct characterization testing of the raw material/ancillary material and prepare a specifications document (e.g. identity, purity, functionality, viral contamination, animal origin) |
✔ |
✔ |
|
Execute a quality and supply agreement |
✔ |
✔ |
|
Provide user requirement specifications to the raw material/ancillary material supplier |
✔ |
|
|
Conduct a risk-based raw material/ancillary material supplier qualification process, generally including initial screening, onsite audit, formalized approval, continuous monitoring/oversight |
✔ |
|
|
Determine if biocompatibility, biodistribution, cytotoxicity or adventitious agent testing is needed (or if testing results are available from the raw material/ancillary material supplier, if applicable) |
✔ |
|
|
Conduct a risk assessment for the use of an AM, based on information provided by the raw material/ancillary material supplier, or in collaboration with the AM supplier, e.g. failure modes and effects analysis |
✔ |
|
|
Establish similar assurances and plans for alternative suppliers |
✔ |
|
|
Qualify the performance of the raw material/ancillary material in the intended application |
✔ |
|
|
Confirm the CoA test result(s) critical to the cell product (e.g. functional assay) |
✔ |
|
|
Assess the effect of lot-to-lot variation of the raw material/ancillary material on the final cell product |
✔ |
|
|
Establish and implement a qualification plan for the use of an raw material/ancillary material |
✔ |
Conclusion
The selection of GMP grade raw materials is a critical step in the manufacturing of cell therapies. These materials not only comply with rigorous quality standards but also support the specific requirements of cell therapy manufacturing processes. By choosing GMP grade raw materials, manufacturers ensure patient safety, regulatory compliance, and the consistent production of high-quality cell therapies. This commitment to quality and efficacy paves the way for the advancement of cell therapy as a transformative field of medicine, offering hope to patients worldwide.