Tips and tricks for iPSC culture
Top tips for successful induced pluripotent stem cell and embryonic stem cell culture.
In the field of regenerative medicine and developmental biology research, the basic technique of culturing pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), is crucial. Nevertheless, keeping PSCs from differentiating can be quite problematic. This article will offer some nuggets of wisdom to aid in the successful and consistent culture of PSCs.
1. Start with High-Quality Cell Culture Vessels and Extracellular Matrices (ECM)
Invest in high-quality cell culture dishes or plates specifically designed for PSCs. Most PSC cultures require extracellular matrices (ECM). Select culture dishes that are compatible with the coating of ECMs like Matrigel, Geltrex, Laminin, fibronectin, etc.
Choose ECMs that are animal component-free to ensure better consistency in the results as well as avoid any contaminants that can potentially come from animal sources.
2. Regularly Inspect and Clean Equipment
To ensure the viability and expansion of your iPSC cultures, maintaining a sterile environment is crucial. Guard against contamination by thoroughly examining and sanitizing key equipment like incubators, hoods, and pipettes. Contaminants, such as bacteria, fungi, or mycoplasma, can jeopardize the integrity of your iPSC cultures. Traces of such impurities, no matter how tiny, may undermine the accuracy of your results or render your research unviable. Properly employing disinfectants and following strict routines is the only way to maintain aseptic conditions.
3. Optimize Media Formulations
Select a defined and reliable PSC culture medium that supports pluripotency. Media should be supplemented with essential components such as growth factors, serum replacement, and small molecules. Growth factors play a major role in maintaining the pluripotency. Choose growth factors derived from optimal expression systems. For example, growth factors derived from a Human expression system are superior to a bacterial system as they have enhanced bioactivity and stability in the cell culture media. Try using animal component-free growth factors to yield better experimental consistency. Ensure that the media and its components are not used post the expiry date.
|
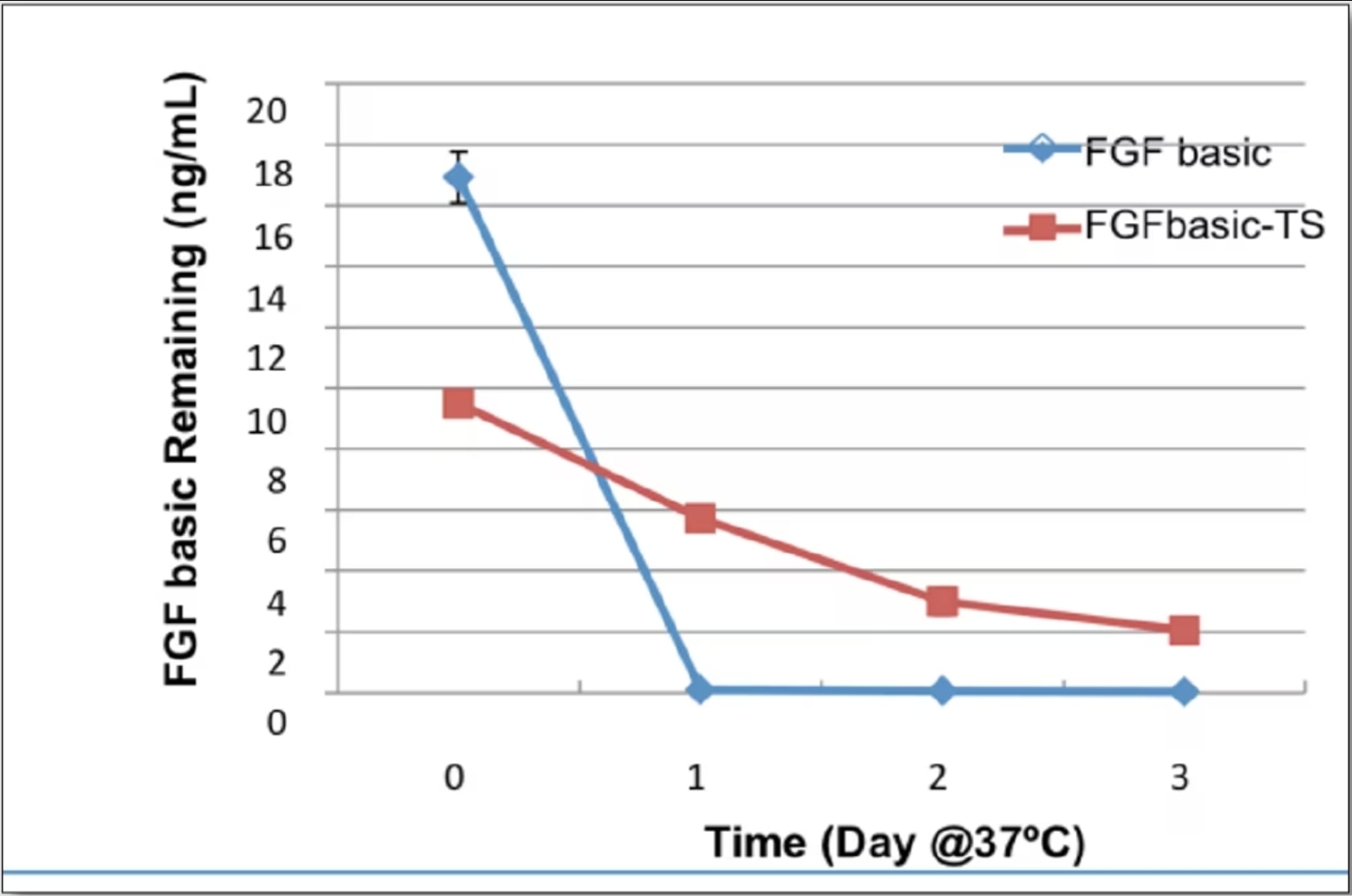
Stability of HumanKine thermostable bFGF-TS in xeno free media w/o cells
|
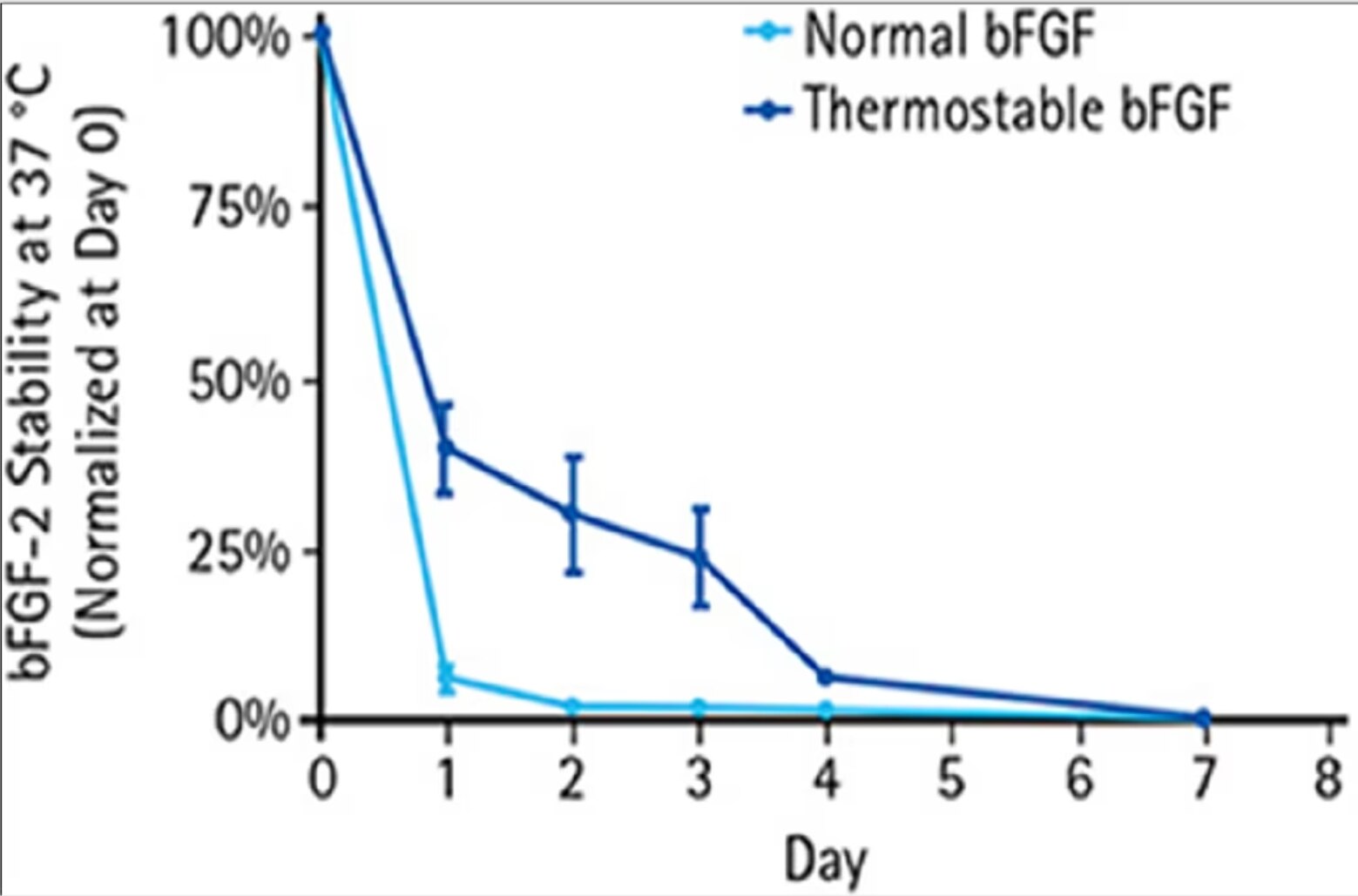
Stability of HumanKine bFGF-TS in iPSC culture compared to normal bFGF By: Nick Asbrock, Christine Chen and Vi Chu*, EMD Millipore, Bioscience Division, Temecula, CA, USA. |
4. Handle Cells Gently
iPSCs are highly sensitive to mechanical stress. Vigorous pipetting or harsh detachment methods, can damage cell membranes, disrupt cell-cell interactions, and lead to a loss of cell viability. When pipetting iPSCs, use wide-bore pipette tips to minimize shear force on the cells. These tips reduce the chances of damaging cells during aspiration and dispensing. When passaging iPSCs, choose a gentle detachment method using EDTA or dispase.
5. Regularly Monitor Cell Density
One of the most important factors in successful PSC cultures is optimal cell density. Too few cells may not be able to absorb enough nutrients, while too many cells can lead to nutrient loss and waste accumulation. Maintaining proper cell density is essential for maintaining their pluripotency. Too many cells can cause differentiation, resulting in lost pluripotency, and unwanted cell types.
6. Use ROCK Inhibitor During Passaging
Using a Rho kinase (ROCK) inhibitor during passaging is a valuable technique, especially when working with sensitive cell types like induced pluripotent stem cells (iPSCs). iPSCs can be vulnerable to stress during passaging, which can lead to cell death. ROCK inhibitors mitigate cell stress, improving cell survival rates during detachment, reseeding, and attachment.
7. Regularly Test for Mycoplasma Contamination
Mycoplasma contamination can often go unnoticed and affect the survivability and pluripotency of PSC cultures. Mycoplasma can negatively impact the differentiation experiments leading to the wastage of valuable reagents and samples. Although there are recommended protocols to clear mycoplasma in cell cultures using some antibiotics, studies have shown mixed results. If you detect mycoplasma in your PSC cultures, it is better to get rid of the infected sample and start fresh.
9. Check Pluripotency Before Starting Differentiations.
Pluripotency is the ability of a cell to differentiate into any of the three cell types in the three layers of the germ: endoderm, mesoderm, or ectoderm. It is important to ensure that your iPSC(s) or ESC(s) are truly pluripotent before differentiation to obtain accurate and meaningful results in subsequent experiments. Pluripotency can be measured by employing specific antibodies to detect pluripotency markers in Immunocytochemistry, flow cytometry, or genetic analysis using qPCR, etc. It is critical to start with at least a 95% pluripotent stem cell population for any differentiations.
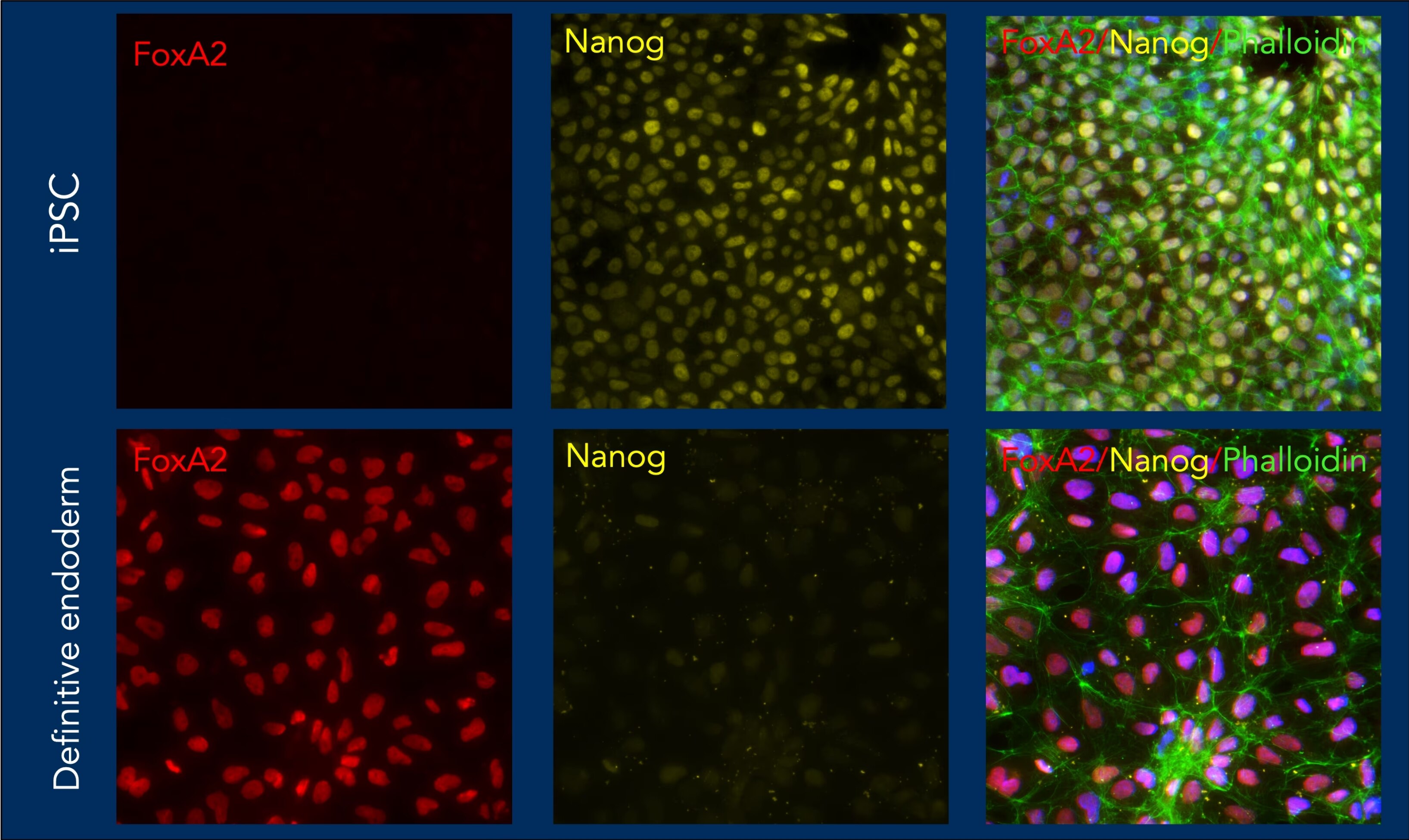
iPSC differentiated to definitive endoderm using HumanKine Animal component free growth factors and characterized using Proteintech antibodies.
9. Maintain Records and Documentation
It is essential to maintain a comprehensive record of your culture conditions and passaging protocols, as well as any observed cell behavior. This documentation is of great value for the resolution of issues and the purpose of reproducibility. Additionally, it is beneficial to stay abreast of the most recent developments in PSC culture methods and technologies. By networking and collaborating with researchers from the field, one can gain valuable knowledge and solutions to common issues.
Conclusion
Culturing pluripotent stem cells is both an art and a science. By following these tips and tricks and maintaining strict adherence to best practices, you can enhance the success and reproducibility of your PSC cultures. Whether you're studying developmental biology, regenerative medicine, or disease modeling, mastering PSC cultures is a critical step toward advancing your research and unlocking the full potential of pluripotent stem cells.
Proteintech reagents for advancing pluripotent stem cell research
Antibodies
Pluripotency marker antibodies |
|||
|
|
Marker |
Proteintech Antibodies |
Number of citations |
|
|
Nanog |
184 |
|
|
|
OCT4 |
197 |
|
|
|
SOX2 |
212 |
|
|
|
KLF4 |
106 |
|
|
|
Endoderm marker antibodies |
||
|
|
Marker |
Proteintech Antibodies |
Number of citations |
|
|
SOX17 |
7 |
|
|
|
FOXA2 |
16 |
|
|
|
CXCR4 |
36 |
|
|
|
Mesoderm marker antibodies |
||
|
|
Marker |
Proteintech Antibodies |
Number of citations |
|
|
NCAM1 |
41 |
|
|
|
NKX 2.5 |
12 |
|
|
|
TBX6 |
|
|
|
|
Ectoderm marker antibodies |
||
|
|
Marker |
Proteintech Antibodies |
Number of citations |
|
|
Nestin |
70 |
|
|
|
PAX6 |
49 |
|
|
|
OTX2 |
22 |
|
HumanKine Growth factors
Pluripotent stem cell(PSC) maintenance |
||
|
Growth factor |
HumanKine |
Number of citations |
|
bFGF |
50 |
|
|
TGFb1 |
156 |
|
|
|
Growth factors for germ layer differentiations |
|
|
Growth factor |
Proteintech products |
Number of citations |
|
Activin A |
25 |
|
|
Noggin |
13 |
|
|
BMP2 |
32 |
|
|
BMP4 |
69 |
|
|
bFGF |
50 |
|
|
|
Growth factors for Neural Differentiation |
|
|
Growth factor |
Proteintech products |
Number of citations |
|
bFGF |
50 |
|
|
EGF |
10 |
|
|
beta NGF |
|
|
|
BDNF |
|
|
|
GDNF |
1 |
|
|
FGF8B |
3 |
|
|
CNTF |
|
|
|
DKK1 |
|
|
|
FGF19 |
1 |
|
|
|
Growth factors for Cardiac differentiation |
|
|
Growth factor |
Proteintech products |
Number of citations |
|
Activin A |
25 |
|
|
BMP4 |
69 |
|
|
bFGF |
50 |
|
|
VEGF165 |
29 |
|
|
VEGF121 |
18 |
|
|
|
Growth factors for Pancreatic differentiation |
|
|
Growth factor |
Proteintech products |
Number of citations |
|
HGF |
14 |
|
|
BMP4 |
69 |
|
|
IGF1 |
|
|
|
Activin A |
25 |
|
|
|
Growth factors for Hepatocyte differentiation |
|
|
Growth factor |
Proteintech products |
Number of citations |
|
bFGF |
50 |
|
|
Wnt3A |
2 |
|
|
HGF |
14 |
|
|
OSM |
5 |
|
|
FGF19 |
|
|
|
Rspondin 1 |
1 |
|
|
BMP4 |
69 |
|
|
EGF |
10 |