How to culture mouse primary cerebral microvascular endothelial cells
Learn to how to isolate and culture these integral cells types for blood brain barrier research.
Written by Arun Flynn, Final-Year PhD student in Cardiovascular Pharmacology, University of Glasgow, UK.
Cerebral endothelial cells are an integral part of the cerebral circulation and differ vastly from the endothelium of systemic blood vessels. For example, they possess unique cell-to-cell junctions, thereby forming a key structural component of the blood-brain barrier (BBB). Nearby cells in the brain such as pericytes, microglia, perivascular macrophages, neurons, and astrocytes support and mediate BBB integrity (referred to as the neurovascular unit) and numerous diseases of the brain are associated with cerebral endothelial dysfunction, loss of BBB integrity, and disruption to cells of the NVU.[1] In particular, the endothelium of the cerebral microvasculature is crucial for maintaining cerebral blood flow and regulating vascular tone, and central to this action of the cerebral endothelium is the production of the vasodilator molecule nitric oxide (NO).[2] It is also well documented that impairment of endothelial NO synthesis underpins numerous cerebrovascular diseases, making the study of cerebral endothelial cells of great importance to researchers.[2] However, appropriately studying the importance of the cerebral endothelium can be challenging without complex and expensive imaging techniques. Thus, researchers often turn to culturing cerebral endothelial cells (e.g., human cerebral microvascular endothelial cells; hCMEC/D3); however, there are many disadvantages to using secondary or “immortalized” endothelial cell lines.
In this blog, we provide a guide on isolating and culturing primary mouse brain microvascular endothelial cells!
Workflow of the isolation and culture of primary mouse brain microvascular endothelial cells.
Note: This technique was originally described by Abbott et al (1992)[3] for use on rat brain tissue. However, it has been amended for use on mice with the aim of achieving an increased cellular yield. As always with tissue culture, keep all components sterile and perform all steps in the hood.
Reagents/equipment needed:
- Working buffer: 500 mL HBSS, 1.2g HEPES, 5mL Pen-Strep, 12mL 22% BSA (see below)
- 22% BSA (First link, cat: 43-20-850)
- Fibronectin stock
- 70 µm cell strainers
- Collagenase I and Collagenase/Dispase (each 50 ng/µL)
- Specialist endothelial cell culture media
- Puromycin stock
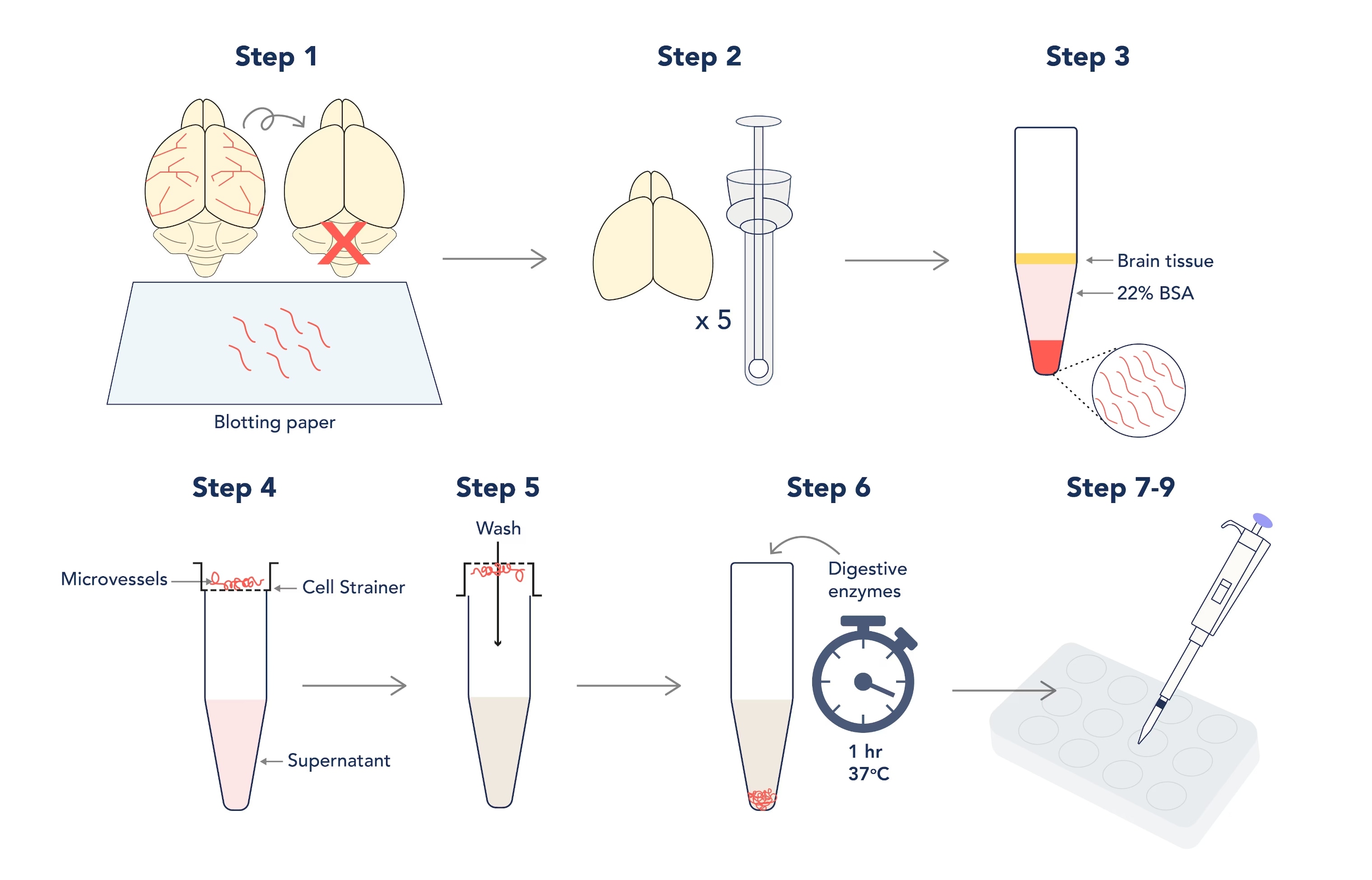
Figure 1. Graphic depiction of the isolation and culture of primary mouse brain microvascular endothelial cells.
- When dissecting the brain, remember to discard the cerebellum and roll the brain over sterile blotting paper to remove the meninges and large cerebral arteries (the aim is to isolate the microvessels within the brain tissue only!).
- Homogenize up to 5 brains in a sterile dowse homogenizer in working buffer; do not use lysis beads! The aim here is to be gentle.
- Pellet your lysate before resuspending in your 22% BSA – this will separate microvessels from fatty brain tissue during long and slow centrifugation steps! However, this step needs 3 or more repeats to maximize the yield.
Note: aim for 20-minute spins at 300 x g
- Pool your vessel pellets and pass the pooled suspension through a cell strainer to separate debris from the vessels that will collect on the strainer.
- Invert the strainer over a fresh tube and collect your vessels with fresh buffer.
- Incubate your resuspended vessels with collagenase I and collagenase/dispase for one hour at 37°C to disrupt the vessel walls (this will increase the surface area for cerebral endothelial cells to culture from). Spin and resuspend digested vessels in culture media.
Note: you must pellet and resuspend the vessels afterwards to prevent total digestion of the tissue!
- Coat wells of a 12- or 24-well plate with Fibronectin (diluted 1:10 in PBS) and incubate for one hour at 37°C before 2x washes with PBS.
Note: Fibronectin is essential for adherence to the wells, and primary cerebral endothelial cells do not culture effectively in larger well sizes!
- Plate out 1mL of vessels per well and allow them to adhere to the fibronectin coating overnight at 37°C. The next day, wash vessels with PBS to remove any remaining debris.
- Replace final PBS wash with endothelial media containing 1µg/mL of Puromycin for 3+ days to achieve 99% homogeneity.
Note: skipping the Puromycin step will allow for co-cultures of cerebral endothelial cells and pericytes/fibroblasts to grow; however, these other cells culture much quicker and may outcompete your cerebral endothelial cells by 7–10 days.
Did you know? Cerebral endothelial cells produce significantly greater amounts of P-glycoprotein than other cells of the neurovascular unit, meaning that it can shuttle Puromycin out of the cell before it becomes cytotoxic! Therefore, we can use Puromycin as a selection tool for endothelial cells over other cell types.[4]
Lastly, here are some suggested applications for your primary cultured cerebral endothelial cells:
- Culture cerebral endothelial cells on fibronectin-coated cover slips for immunofluorescence experiments.
- Lyse cells for protein for Western blotting.
- Extract RNA for RT-qPCR or transcriptomics analysis (such as RNAseq).
Antibodies to study the neurovascular unit
|
Tight junctions |
Transporter proteins |
Others |
|
Neuron markers |
Astrocyte markers |
Microglia markers |
Pericyte markers |
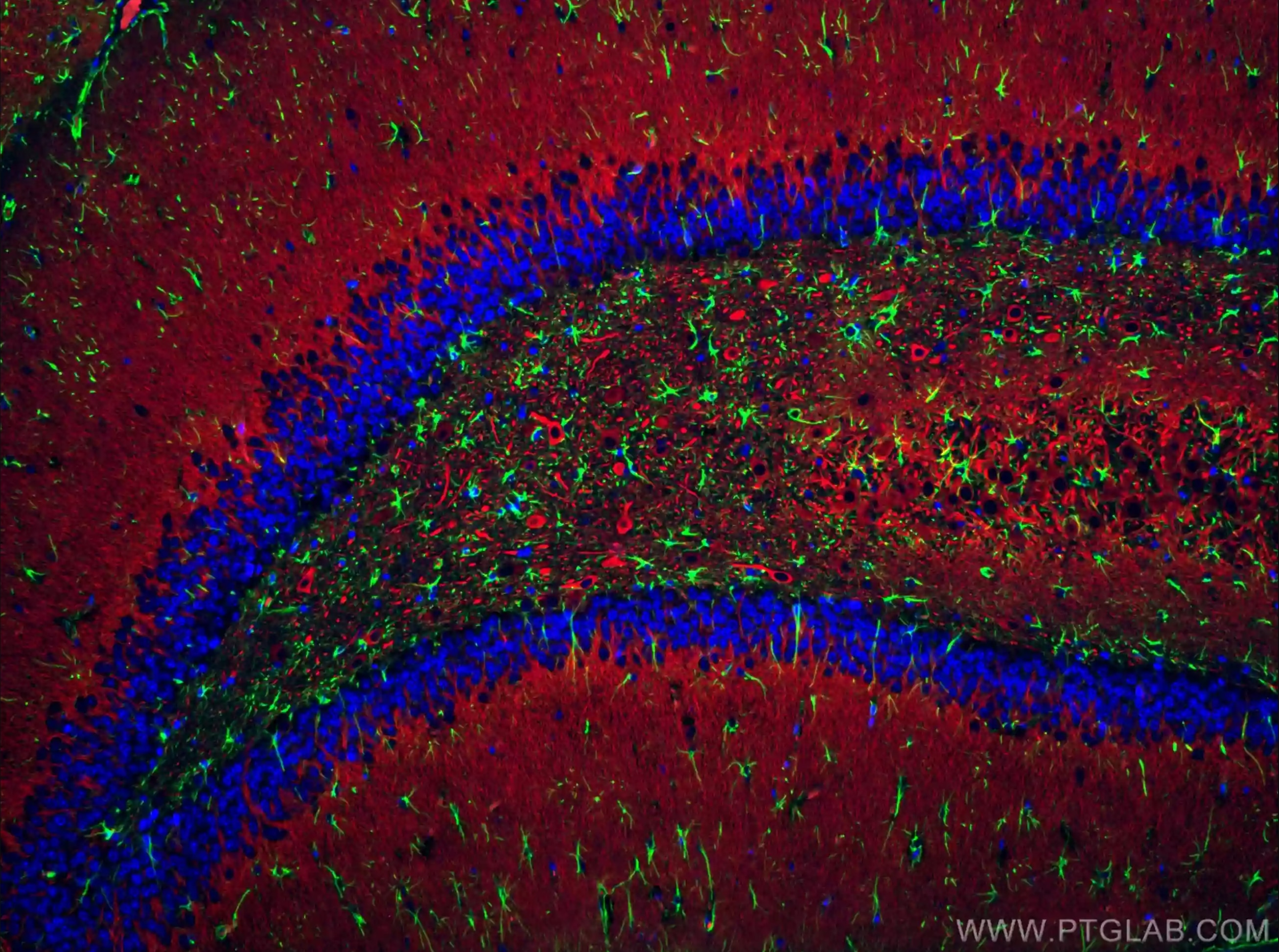
Immunofluorescent analysis of (4% PFA) fixed rat brain tissue using CoraLite® Plus 488 GFAP antibody (CL488-16825) at dilution of 1:200, CoraLite®594 MAP2 antibody (CL594-17490, red). DAPI (blue).
References
[1] Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011 Nov 3;12(12):723-38. doi: 10.1038/nrn3114.
[2] Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1566-82. doi: 10.1152/ajpheart.01310.2010.
[3] Abbott NJ, Hughes CCW, Revest PA, J. Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci 1 September 1992; 103 (1): 23–37. doi: 10.1242/jcs.103.1.23
[4] Greenwood J. Characterization of a rat retinal endothelial cell culture and the expression of P-glycoprotein in brain and retinal endothelium in vitro. J Neuroimmunol. 1992 Jul;39(1-2):123-32. doi: 10.1016/0165-5728(92)90181-j.